Enamel: Molecular identity of its transepithelial ion transport system
- PMID: 28389033
- PMCID: PMC5944837
- DOI: 10.1016/j.ceca.2017.03.006
Enamel: Molecular identity of its transepithelial ion transport system
Abstract
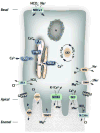
Enamel is the most calcified tissue in vertebrates. It differs from bone in a number of characteristics including its origin from ectodermal epithelium, lack of remodeling capacity by the enamel forming cells, and absence of collagen. The enamel-forming cells known as ameloblasts, choreograph first the synthesis of a unique protein-rich matrix, followed by the mineralization of this matrix into a tissue that is ∼95% mineral. To do this, ameloblasts arrange the coordinated movement of ions across a cell barrier while removing matrix proteins and monitoring extracellular pH using a variety of buffering systems to enable the growth of carbonated apatite crystals. Although our knowledge of these processes and the molecular identity of the proteins involved in transepithelial ion transport has increased in the last decade, it remains limited compared to other cells. Here we present an overview of the evolution and development of enamel, its differences with bone, and describe the ion transport systems associated with ameloblasts.
Keywords: CRAC channel; Enamel; Ion transport; pH.
Copyright © 2017 The Author. Published by Elsevier Ltd.. All rights reserved.
Figures

References
-
- Smith CE. Cellular and chemical events during enamel maturation. Crit Rev Oral Biol Med. 1998;9(2):128–61. - PubMed
-
- Boyde A. Microstructure of enamel. Ciba Found Symp. 1997;205:18–27. discussion 27–31. - PubMed
-
- Hubbard MJ. Abundant calcium homeostasis machinery in rat dental enamel cells. Up-regulation of calcium store proteins during enamel mineralization implicates the endoplasmic reticulum in calcium transcytosis. Eur J Biochem. 1996;239(3):611–23. - PubMed
-
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1(1):11–21. - PubMed
-
- Hubbard MJ. Calcium transport across the dental enamel epithelium. Crit Rev Oral Biol Med. 2000;11(4):437–66. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

