Brachyury drives formation of a distinct vascular branchpoint critical for fetal-placental arterial union in the mouse gastrula
- PMID: 28389228
- PMCID: PMC5760991
- DOI: 10.1016/j.ydbio.2017.03.032
Brachyury drives formation of a distinct vascular branchpoint critical for fetal-placental arterial union in the mouse gastrula
Abstract
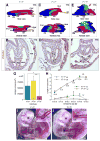
How the fetal-placental arterial connection is made and positioned relative to the embryonic body axis, thereby ensuring efficient and directed blood flow to and from the mother during gestation, is not known. Here we use a combination of genetics, timed pharmacological inhibition in living mouse embryos, and three-dimensional modeling to link two novel architectural features that, at present, have no status in embryological atlases. The allantoic core domain (ACD) is the extraembryonic extension of the primitive streak into the allantois, or pre-umbilical tissue; the vessel of confluence (VOC), situated adjacent to the ACD, is an extraembryonic vessel that marks the site of fetal-placental arterial union. We show that genesis of the fetal-placental connection involves the ACD and VOC in a series of steps, each one dependent upon the last. In the first, Brachyury (T) ensures adequate extension of the primitive streak into the allantois, which in turn designates the allantoic-yolk sac junction. Next, the streak-derived ACD organizes allantoic angioblasts to the axial junction; upon signaling from Fibroblast Growth Factor Receptor-1 (FGFR1), these endothelialize and branch, forming a sprouting VOC that unites the umbilical and omphalomesenteric arteries with the fetal dorsal aortae. Arterial union is followed by the appearance of the medial umbilical roots within the VOC, which in turn designate the correct axial placement of the lateral umbilical roots/common iliac arteries. In addition, we show that the ACD and VOC are conserved across Placentalia, including humans, underscoring their fundamental importance in mammalian biology. We conclude that T is required for correct axial positioning of the VOC via the primitive streak/ACD, while FGFR1, through its role in endothelialization and branching, further patterns it. Together, these genetic, molecular and structural elements safeguard the fetus against adverse outcomes that can result from vascular mispatterning of the fetal-placental arterial connection.
Keywords: Allantoic core domain (ACD); Brachyury (T); Fibroblast Growth Factor Receptor 1 (FGFR1); Primitive streak; Vascular patterning; Vessel of confluence (VOC).
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures





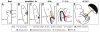
References
-
- Beddington RSP. Induction of a second neural axis by the mouse node. Development. 1994;120:613–620. - PubMed
-
- Blum M, Andre P, Muders K, Schweickert A, Fischer A, Bitzer E, Bogusch S, Beyer T, van Straaten HW, Viebahn C. Ciliation and gene expression distinguish between node and posterior notochord in the mammalian embryo. Differ Res Biol Divers. 2007;75:133–146. - PubMed
-
- Bonnevie K. New facts on mesoderm formation and proamnion derivatives in the normal mouse embryo. J Morphol. 1950;86:495–546. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

