Rationale and Design of Khuzestan Vitamin D Deficiency Screening Program in Pregnancy: A Stratified Randomized Vitamin D Supplementation Controlled Trial
- PMID: 28389419
- PMCID: PMC5400884
- DOI: 10.2196/resprot.7159
Rationale and Design of Khuzestan Vitamin D Deficiency Screening Program in Pregnancy: A Stratified Randomized Vitamin D Supplementation Controlled Trial
Abstract
Background: Although there have been marked improvements in our understanding of vitamin D functions in different diseases, gaps on its role during pregnancy remain. Due to the lack of consensus on the most accurate marker of vitamin D deficiency during pregnancy and the optimal level of 25-hydroxyvitamin D, 25(OH)D, for its definition, vitamin D deficiency assessment during pregnancy is a complicated process. Besides, the optimal protocol for treatment of hypovitaminosis D and its effect on maternal and neonatal outcomes are still unclear.
Objective: The aim of our study was to estimate the prevalence of vitamin D deficiency in the first trimester of pregnancy and to compare vitamin D screening strategy with no screening. Also, we intended to compare the effectiveness of various treatment regimens on maternal and neonatal outcomes in Masjed-Soleyman and Shushtar cities of Khuzestan province, Iran.
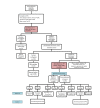
Methods: This was a two-phase study. First, a population-based cross-sectional study was conducted; recruiting 1600 and 900 first trimester pregnant women from health centers of Masjed-Soleyman and Shushtar, respectively, using stratified multistage cluster sampling with probability proportional to size (PPS) method. Second, to assess the effect of screening strategy on maternal and neonatal outcomes, Masjed-Soleyman participants were assigned to a screening program versus Shushtar participants who became the nonscreening arm. Within the framework of the screening regimen, an 8-arm blind randomized clinical trial was undertaken to compare the effects of various treatment protocols. A total of 800 pregnant women with vitamin D deficiency were selected using simple random sampling from the 1600 individuals of Masjed-Soleyman as interventional groups. Serum concentrations of 25(OH)D were classified as: (1) severe deficient (<10ng/ml), (2) moderate deficient (10-20ng/ml), and (3) normal status (>20ng/ml). Those with severe and moderate deficiency were randomly divided into 4 subgroups and received vitamin D3 based on protocol and were followed until delivery. Data was analyzed according to the intention-to-treat principle.
Results: Recruitment commenced in July, 2014, and as estimated, nearly 3.5 years is needed to complete the study. Results of this study will (1) provide reliable information regarding the prevalence of vitamin D deficiency during pregnancy using universal vitamin D screening approach and (2) determine the beneficial effects of universal screening and compare the various treatment protocols in terms of pregnancy outcomes.
Conclusions: Since vitamin D deficiency is a prevalent disorder in pregnancy among Iranian population, this study will ensure creation of reliable evidence-based findings and will enable clinicians to better evaluate and treat vitamin D deficient pregnant women.
Trial registration: International Standard Randomized Controlled Trial Number (ISRCTN): 2014102519660N1; http://www.irct.ir/searchresult.php?keyword=&id=19660&number=1&prt=7805&total=10&m=1 (Archived by WebCite at http://www.webcitation.org/6p3lkqFdV).
Keywords: clinical trial; pregnancy; vitamin D deficiency.
©Maryam Rostami, Fahimeh Ramezani Tehrani, Masoumeh Simbar, Farhad Hosseinpanah, Hamid Alavi Majd. Originally published in JMIR Research Protocols (http://www.researchprotocols.org), 07.04.2017.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
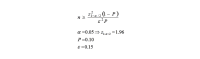
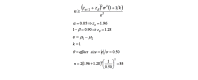
References
-
- Nair R, Maseeh A. Vitamin D: The “sunshine” vitamin. J Pharmacol Pharmacother. 2012 Apr;3(2):118–26. doi: 10.4103/0976-500X.95506. http://www.jpharmacol.com/article.asp?issn=0976-500X;year=2012;volume=3;... - DOI - PMC - PubMed
-
- Kikuta J, Ishii M. [Current Topics on Vitamin D. The effects of vitamin D on the immune system] Clin Calcium. 2015 Mar;25(3):359–65. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

