New Insights into PhaM-PhaC-Mediated Localization of Polyhydroxybutyrate Granules in Ralstonia eutropha H16
- PMID: 28389545
- PMCID: PMC5452819
- DOI: 10.1128/AEM.00505-17
New Insights into PhaM-PhaC-Mediated Localization of Polyhydroxybutyrate Granules in Ralstonia eutropha H16
Abstract
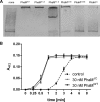
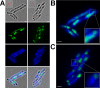
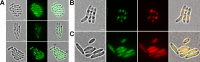
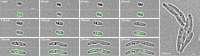
The formation and localization of polyhydroxybutyrate (PHB) granules in Ralstonia eutropha are controlled by PhaM, which interacts both with the PHB synthase (PhaC) and with the bacterial nucleoid. Here, we studied the importance of proline and lysine residues of two C-terminal PAKKA motifs in PhaM for their importance in attaching PHB granules to DNA by in vitro and in vivo methods. Substitution of the lysine residues but not of the proline residues resulted in detachment of formed PHB granules from the nucleoid. Instead, formation of PHB granule clusters at polar regions of the rod-shaped cells and an unequal distribution of PHB granules to daughter cells were observed. The formation of PHB granules was studied by the expression of chromosomally anchored gene fusions of fluorescent proteins with PhaM and PhaC in different backgrounds. PhaM and PhaC fusions showed a distinct colocalization at formed PHB granules in the nucleoid region of the wild type. In a ΔphaC background, PhaM and the catalytically inactive PhaCC319A protein were not able to form fluorescent foci, indicating that correct positioning requires the formation of PHB. Furthermore, time-lapse experiments revealed that PhaC and PhaM proteins detach from formed PHB granules at later stages, resulting in a nonhomogeneous population of PHB granules. This could explain why growth of individual PHB granules stops under PHB-permissive conditions at a certain size.IMPORTANCE PHB granules are storage compounds for carbon and energy in many prokaryotes. Equal distribution of accumulated PHB granules during cell division is therefore important for optimal fitness of the daughter cells. In R. eutropha, PhaM is responsible for maximal activity of PHB synthase, for initiation of PHB granule formation at discrete regions in the cells, and for association of formed PHB granules with the nucleoid. Here we found that four lysine residues of C-terminal PhaM sequence motifs are essential for association of PHB granules with the nucleoid. Furthermore, we followed PHB granule formation by time-lapse microscopy and provide evidence for aging of PHB granules that is manifested by detachment of previously PHB granule-associated PhaM and PHB synthase.
Keywords: PHB accumulation; Ralstonia; Ralstonia eutropha; biopolymer.
Copyright © 2017 American Society for Microbiology.
Figures




References
-
- Pötter M, Steinbüchel A. 2006. Biogenesis and structure of polyhydroxyalkanoate granules. Microbiol Monogr 1:1–28.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

