Control of Kir channel gating by cytoplasmic domain interface interactions
- PMID: 28389584
- PMCID: PMC5412532
- DOI: 10.1085/jgp.201611719
Control of Kir channel gating by cytoplasmic domain interface interactions
Abstract
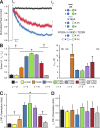
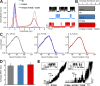
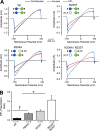
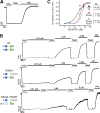
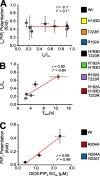
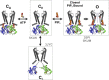
Inward rectifier potassium (Kir) channels are expressed in almost all mammalian tissues and play critical roles in the control of excitability. Pancreatic ATP-sensitive K (KATP) channels are key regulators of insulin secretion and comprise Kir6.2 subunits coupled to sulfonylurea receptors. Because these channels are reversibly inhibited by cytoplasmic ATP, they link cellular metabolism with membrane excitability. Loss-of-function mutations in the pore-forming Kir6.2 subunit cause congenital hyperinsulinism as a result of diminished channel activity. Here, we show that several disease mutations, which disrupt intersubunit salt bridges at the interface of the cytoplasmic domains (CD-I) of adjacent subunits, induce loss of channel activity via a novel channel behavior: after ATP removal, channels open but then rapidly inactivate. Re-exposure to inhibitory ATP causes recovery from this inactivation. Inactivation can be abolished by application of phosphatidylinositol-4,5-bisphosphate (PIP2) to the cytoplasmic face of the membrane, an effect that can be explained by a simple kinetic model in which PIP2 binding competes with the inactivation process. Kir2.1 channels contain homologous salt bridges, and we find that mutations that disrupt CD-I interactions in Kir2.1 also reduce channel activity and PIP2 sensitivity. Kir2.1 channels also contain an additional CD-I salt bridge that is not present in Kir6.2 channels. Introduction of this salt bridge into Kir6.2 partially rescues inactivating mutants from the phenotype. These results indicate that the stability of the intersubunit CD-I is a major determinant of the inactivation process in Kir6.2 and may control gating in other Kir channels.
© 2017 Borschel et al.
Figures




References
-
- Amorós I., Dolz-Gaitón P., Gómez R., Matamoros M., Barana A., de la Fuente M.G., Núñez M., Pérez-Hernández M., Moraleda I., Gálvez E., et al. . 2013. Propafenone blocks human cardiac Kir2.x channels by decreasing the negative electrostatic charge in the cytoplasmic pore. Biochem. Pharmacol. 86:267–278. 10.1016/j.bcp.2013.04.023 - DOI - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

