Intravascular donor monocytes play a central role in lung transplant ischaemia-reperfusion injury
- PMID: 28389600
- PMCID: PMC5870457
- DOI: 10.1136/thoraxjnl-2016-208977
Intravascular donor monocytes play a central role in lung transplant ischaemia-reperfusion injury
Abstract
Rationale: Primary graft dysfunction in lung transplant recipients derives from the initial, largely leukocyte-dependent, ischaemia-reperfusion injury. Intravascular lung-marginated monocytes have been shown to play key roles in experimental acute lung injury, but their contribution to lung ischaemia-reperfusion injury post transplantation is unknown.
Objective: To define the role of donor intravascular monocytes in lung transplant-related acute lung injury and primary graft dysfunction.
Methods: Isolated perfused C57BL/6 murine lungs were subjected to warm ischaemia (2 hours) and reperfusion (2 hours) under normoxic conditions. Monocyte retention, activation phenotype and the effects of their depletion by intravenous clodronate-liposome treatment on lung inflammation and injury were determined. In human donor lung transplant samples, the presence and activation phenotype of monocytic cells (low side scatter, 27E10+, CD14+, HLA-DR+, CCR2+) were evaluated by flow cytometry and compared with post-implantation lung function.
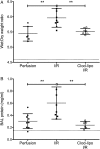
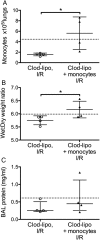
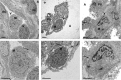
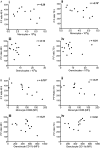
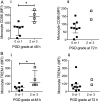
Results: In mouse lungs following ischaemia-reperfusion, substantial numbers of lung-marginated monocytes remained within the pulmonary microvasculature, with reduced L-selectin and increased CD86 expression indicating their activation. Monocyte depletion resulted in reductions in lung wet:dry ratios, bronchoalveolar lavage fluid protein, and perfusate levels of RAGE, MIP-2 and KC, while monocyte repletion resulted in a partial restoration of the injury. In human lungs, correlations were observed between pre-implantation donor monocyte numbers/their CD86 and TREM-1 expression and post-implantation lung dysfunction at 48 and 72 hours.
Conclusions: These results indicate that lung-marginated intravascular monocytes are retained as a 'passenger' leukocyte population during lung transplantation, and play a key role in the development of transplant-associated ischaemia-reperfusion injury.
Keywords: Innate Immunity; Lung Transplantation; Macrophage Biology.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Competing interests: None declared.
Figures


Comment in
-
Donor intravascular monocyte trafficking: a potential therapeutic target for primary graft dysfunction following lung transplantation?Thorax. 2018 Apr;73(4):303-304. doi: 10.1136/thoraxjnl-2017-210274. Epub 2018 Jan 31. Thorax. 2018. PMID: 29386299 No abstract available.
Similar articles
-
The depletion of donor macrophages reduces ischaemia-reperfusion injury after mouse lung transplantation.Eur J Cardiothorac Surg. 2014 Apr;45(4):703-9. doi: 10.1093/ejcts/ezt489. Epub 2013 Oct 10. Eur J Cardiothorac Surg. 2014. PMID: 24113322
-
Depletion of alveolar macrophages by clodronate-liposomes aggravates ischemia-reperfusion injury of the lung.J Heart Lung Transplant. 2005 Jan;24(1):38-45. doi: 10.1016/j.healun.2003.10.007. J Heart Lung Transplant. 2005. PMID: 15653377
-
Tissue-derived proinflammatory effect of adenosine A2B receptor in lung ischemia-reperfusion injury.J Thorac Cardiovasc Surg. 2010 Oct;140(4):871-7. doi: 10.1016/j.jtcvs.2010.06.051. Epub 2010 Jul 24. J Thorac Cardiovasc Surg. 2010. PMID: 20659747 Free PMC article.
-
Update of early respiratory failure in the lung transplant recipient.Curr Opin Crit Care. 2006 Feb;12(1):19-24. doi: 10.1097/01.ccx.0000198995.44943.63. Curr Opin Crit Care. 2006. PMID: 16394779 Review.
-
The effects of ischaemic conditioning on lung ischaemia-reperfusion injury.Respir Res. 2022 Dec 16;23(1):351. doi: 10.1186/s12931-022-02288-z. Respir Res. 2022. PMID: 36527070 Free PMC article. Review.
Cited by
-
Rho-inhibiting C2IN-C3 fusion toxin inhibits chemotactic recruitment of human monocytes ex vivo and in mice in vivo.Arch Toxicol. 2018 Jan;92(1):323-336. doi: 10.1007/s00204-017-2058-y. Epub 2017 Sep 18. Arch Toxicol. 2018. PMID: 28924833 Free PMC article.
-
Primary graft dysfunction after heart transplantation: a thorn amongst the roses.Heart Fail Rev. 2019 Sep;24(5):805-820. doi: 10.1007/s10741-019-09794-1. Heart Fail Rev. 2019. PMID: 31020451 Free PMC article. Review.
-
Senescence in Pulmonary Fibrosis: Between Aging and Exposure.Front Med (Lausanne). 2020 Nov 12;7:606462. doi: 10.3389/fmed.2020.606462. eCollection 2020. Front Med (Lausanne). 2020. PMID: 33282895 Free PMC article. Review.
-
Donor leukocyte trafficking during human ex vivo lung perfusion.Clin Transplant. 2022 Jul;36(7):e14670. doi: 10.1111/ctr.14670. Epub 2022 Apr 18. Clin Transplant. 2022. PMID: 35396887 Free PMC article.
-
Rate of recipient-derived alveolar macrophage development and major histocompatibility complex cross-decoration after lung transplantation in humans.Am J Transplant. 2022 Feb;22(2):574-587. doi: 10.1111/ajt.16812. Epub 2021 Sep 7. Am J Transplant. 2022. PMID: 34431221 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
