RNA sequence analysis of rat acute experimental pancreatitis with and without fatty liver: a gene expression profiling comparative study
- PMID: 28389636
- PMCID: PMC5429720
- DOI: 10.1038/s41598-017-00821-5
RNA sequence analysis of rat acute experimental pancreatitis with and without fatty liver: a gene expression profiling comparative study
Abstract
Fatty liver (FL) is one of the risk factors for acute pancreatitis and is also indicative of a worse prognosis as compared to acute pancreatitis without fatty liver (AP). The aim of the present study was to analyze, at the hepatic level, the differentially expressed genes (DEGs) between acute pancreatitis with fatty liver (APFL) rats and AP rats. GO (Gene Ontology) and KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway analyses of these DEGs indicated that PPARα signalling pathway and fatty acid degradation pathway may be involved in the pathological process of APFL, which indicated that fatty liver may aggravate pancreatitis through these pathways. Moreover, the excessive activation of JAK/STAT signaling pathway and toll-like receptor signaling pathway was also found in APFL group as shown in heat map. In conclusion, the inhibition of PPARα signaling pathway and the fatty acid degradation pathway may lead to the further disorder of lipid metabolism, which can aggravate pancreatitis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


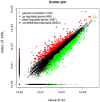




 ), JAK/STAT signaling pathway were marked with an asterisk (
), JAK/STAT signaling pathway were marked with an asterisk ( ), endoplasmic reticulum stress were marked with an asterisk (
), endoplasmic reticulum stress were marked with an asterisk ( ), chemokine receptors were marked with an asterisk (
), chemokine receptors were marked with an asterisk ( ), tumor necrosis factor receptor superfamily were marked with an asterisk (
), tumor necrosis factor receptor superfamily were marked with an asterisk ( ), interleukin were marked with an asterisk (
), interleukin were marked with an asterisk ( ) and toll-like receptors were marked with an asterisk (
) and toll-like receptors were marked with an asterisk ( ).
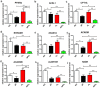
).

Similar articles
-
A High Phosphorus Diet Affects Lipid Metabolism in Rat Liver: A DNA Microarray Analysis.PLoS One. 2016 May 17;11(5):e0155386. doi: 10.1371/journal.pone.0155386. eCollection 2016. PLoS One. 2016. PMID: 27187182 Free PMC article.
-
Genomic expression profiling and bioinformatics analysis on diabetic nephrology with ginsenoside Rg3.Mol Med Rep. 2016 Aug;14(2):1162-72. doi: 10.3892/mmr.2016.5349. Epub 2016 May 27. Mol Med Rep. 2016. PMID: 27279428 Free PMC article.
-
Screening and validation of differentially expressed extracellular miRNAs in acute pancreatitis.Mol Med Rep. 2017 Nov;16(5):6412-6418. doi: 10.3892/mmr.2017.7374. Epub 2017 Aug 28. Mol Med Rep. 2017. PMID: 28849189
-
Oxymatrine attenuates hepatic steatosis in non-alcoholic fatty liver disease rats fed with high fructose diet through inhibition of sterol regulatory element binding transcription factor 1 (Srebf1) and activation of peroxisome proliferator activated receptor alpha (Pparα).Eur J Pharmacol. 2013 Aug 15;714(1-3):89-95. doi: 10.1016/j.ejphar.2013.06.013. Epub 2013 Jun 18. Eur J Pharmacol. 2013. PMID: 23791610
-
Global transcriptome‑wide analysis of the function of GDDR in acute gastric lesions.Mol Med Rep. 2017 Dec;16(6):8673-8684. doi: 10.3892/mmr.2017.7687. Epub 2017 Oct 2. Mol Med Rep. 2017. PMID: 28990076 Free PMC article.
Cited by
-
Use of Network Pharmacology to Investigate the Mechanism by Which Allicin Ameliorates Lipid Metabolism Disorder in HepG2 Cells.Evid Based Complement Alternat Med. 2021 Jan 12;2021:3956504. doi: 10.1155/2021/3956504. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 33505493 Free PMC article.
-
Fatty Liver Disease and Non-Alcoholic Fatty Liver Disease Worsen the Outcome in Acute Pancreatitis: A Systematic Review and Meta-Analysis.J Clin Med. 2020 Aug 20;9(9):2698. doi: 10.3390/jcm9092698. J Clin Med. 2020. PMID: 32825458 Free PMC article. Review.
-
Metabolic-associated fatty liver disease is associated with acute pancreatitis with more severe course: Post hoc analysis of a prospectively collected international registry.United European Gastroenterol J. 2023 May;11(4):371-382. doi: 10.1002/ueg2.12389. Epub 2023 Apr 16. United European Gastroenterol J. 2023. PMID: 37062947 Free PMC article.
-
Ameliorative effect of Sedum sarmentosum Bunge extract on Tilapia fatty liver via the PPAR and P53 signaling pathway.Sci Rep. 2018 May 31;8(1):8456. doi: 10.1038/s41598-018-26084-2. Sci Rep. 2018. PMID: 29855491 Free PMC article.
-
Relationships between Metabolic Comorbidities and Occurrence, Severity, and Outcomes in Patients with Acute Pancreatitis: A Narrative Review.Biomed Res Int. 2019 Oct 7;2019:2645926. doi: 10.1155/2019/2645926. eCollection 2019. Biomed Res Int. 2019. PMID: 31687382 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

