Long-Term Kinetics of Immunologic Components and Neurological Deficits in Rats Following Repetitive Mild Traumatic Brain Injury
- PMID: 28390198
- PMCID: PMC5395134
- DOI: 10.12659/msm.901124
Long-Term Kinetics of Immunologic Components and Neurological Deficits in Rats Following Repetitive Mild Traumatic Brain Injury
Abstract
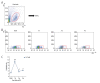
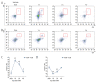
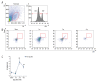
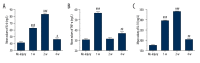
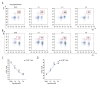
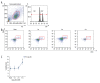
BACKGROUND Despite growing awareness of repetitive mild traumatic brain injury (rmTBI), understanding of the involvement of long-term kinetics of immunologic components in the central and peripheral immune system took part remains incomplete. The present study aimed to provide a quantitative assay for certain immune system parameters in rmTBI rats. MATERIAL AND METHODS Neurological functions were assessed by modified Neurological Severity Score (mNSS) and Morris Water Maze (MWM), immunologic components from brain and peripheral blood were analyzed by flow cytometry (FCM), and concentrations of inflammatory cytokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-10 were measure by enzyme-linked immunosorbent assay (ELISA). RESULTS Neurological functions of rmTBI rats were seriously impaired. In the brain, T cells were up-regulated and peaked at week 1. The percentage of CD4+ T cells decreased from week 1 to week 4, while CD8+ T cells notably decreased at week 1, then increased until week 4. The infiltration proportion of Treg cells was reduced at week 1 and peaked at week 2. CD86+/CD11b+ M1 peaked at week 4 and CD206+/CD11b+ M2 rose at week 1. IL-6/IL-10 showed a similar pattern, whose rise corresponded to the decrease in TNF-α at week 2 after rmTBI. FCM demonstrated peripheral immune dysfunction after rmTBI. CONCLUSIONS mNSS and MWM demonstrated neuronal deficits in rmTBI rats, and central and peripheral immune systems were implicated in the pathophysiological processes of rmTBI. Long-term immune response may play dual roles in injury and repair of rmTBI.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



References
-
- Alves OL, Bullock R. Excitotoxic damage in traumatic brain injury. In: Clark RS, Kochanek P, editors. Brain injury. Boston: Kluwer Academic Publishers; 2001. p. 1.
-
- Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–41. - PubMed
-
- Carli P, Orliaguet G. Severe traumatic brain injury in children. Lancet. 2004;363:584–85. - PubMed
-
- Collins C, Dean J. Acquired brain injury. In: Turner A, Foster M, Johnson SE, editors. Occupational therapy and physical dysfunction: Principles, skills and practice. Edinburgh: Churchill Livingstone; 2002. pp. 395–96.
-
- Hardman JM, Manoukian A. Pathology of head trauma. Neuroimaging Clin N Am. 2002;12:175–87. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

