Fluorescence properties of doxorubicin in PBS buffer and PVA films
- PMID: 28390260
- PMCID: PMC5859936
- DOI: 10.1016/j.jphotobiol.2017.03.024
Fluorescence properties of doxorubicin in PBS buffer and PVA films
Abstract
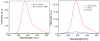
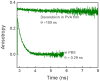
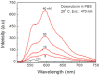
We studied steady-state and time-resolved fluorescence properties of an anticancer drug Doxorubicin in a saline buffer and poly-vinyl alcohol (PVA) film. Absorption of Doxorubicin, located at blue-green spectral region, allows a convenient excitation with visible light emitting diodes or laser diodes. Emission of Doxorubicin with maximum near 600nm can be easily detected with photomultipliers and CCD cameras. Both, absorption and fluorescence spectra in polymeric matrix show more pronounced vibronic structures than in solution. Also, the steady-state anisotropy in the polymer film is significantly higher than in the saline solution. In PVA film the fluorescence anisotropy is about 0.30 whereas in the saline buffer only 0.07. Quantum efficiencies of Doxorubicin were compared to a known standard Rhodamine 101 which has fluorescence emission in a similar spectral region. The quantum yield of Doxorubicin in PVA film is more than 10% and about twice higher than in the saline solution. Similarly, the lifetime of doxorubicin in PVA film is about 2ns whereas in the saline solution only about 1ns. The fluorescence anisotropy decays show that Doxorubicin molecules are freely rotating in the saline buffer with a correlation time of about 290ps, and are almost completely immobilized in the PVA film. The spectroscopic investigations presented in this manuscript are important, as they provide answers to changes in molecular properties of Doxorubicin depending changes in the local environment, which is useful when synthesizing nanoparticles for Doxorubicin entrapment.
Copyright © 2017 Elsevier B.V. All rights reserved.
Figures

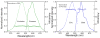

References
-
- Changenet-Barret P, Gustavsson T, Markovitsi D, Manet I, Monti S. Unravelling molecular mechanisms in the fluorescence spectra of doxorubicin in aqueous solution by femtosecond fluorescence spectroscopy. Phys Chem Chem Phys. 2013;15(8):2937–2944. - PubMed
-
- Karukstis KK, Thompson EH, Whiles JA, Rosenfeld RJ. Deciphering the fluorescence signature of daunomycin and doxorubicin. Biophys Chem. 1998;73(3):249–263. - PubMed
-
- Duray PH, Cuono CB, Madri JA. Demonstration of cutaneous doxorubicin extravasation by rhodamine-filtered fluorescence microscopy. J Surg Oncol. 1986;31(1):21–25. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

