Deciphering Neural Codes of Memory during Sleep
- PMID: 28390699
- PMCID: PMC5434457
- DOI: 10.1016/j.tins.2017.03.005
Deciphering Neural Codes of Memory during Sleep
Abstract
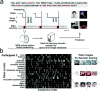
Memories of experiences are stored in the cerebral cortex. Sleep is critical for the consolidation of hippocampal memory of wake experiences into the neocortex. Understanding representations of neural codes of hippocampal-neocortical networks during sleep would reveal important circuit mechanisms in memory consolidation and provide novel insights into memory and dreams. Although sleep-associated ensemble spike activity has been investigated, identifying the content of memory in sleep remains challenging. Here we revisit important experimental findings on sleep-associated memory (i.e., neural activity patterns in sleep that reflect memory processing) and review computational approaches to the analysis of sleep-associated neural codes (SANCs). We focus on two analysis paradigms for sleep-associated memory and propose a new unsupervised learning framework ('memory first, meaning later') for unbiased assessment of SANCs.
Keywords: functional imaging; memory consolidation; memory replay; neural representation; population decoding; sleep-associated memory.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures



References
-
- Andersen P, et al. The Hippocampus Book. Oxford University Press; 2006.
-
- Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–1278. - PubMed
-
- Marshall L, Born J. Contribution of sleep to hippocampus-dependent memory consolidation. Trends Cog Sci. 2007;11:442–450. - PubMed
-
- Buzsaki G. Memory consolidation during sleep: a neurophysiology perspective. J Sleep Res. 1998;7:17–23. - PubMed
-
- Breton J, Robertson EM. Memory processing: The critical role of neuronal replay during sleep. Curr Biol. 2013;23:R836–R838. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

