Measuring nitrate reductase activity from human and rodent tongues
- PMID: 28390999
- PMCID: PMC5484083
- DOI: 10.1016/j.niox.2017.04.001
Measuring nitrate reductase activity from human and rodent tongues
Abstract
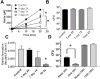
Reduction of salivary nitrate to nitrite by oral microbes expressing nitrate-reductase has emerged as a crucial pathway in systemic NO homeostasis in humans and other mammals. Selective depletion of oral microbes prevents dietary nitrate-dependent lowering of blood pressure, inhibition of platelet aggregation and ischemic injury. To date, most studies interrogate enterosalivary nitrate reduction by following changes in saliva or plasma nitrite and NO-signaling (functional) end points. Little is known about whether, and if so how, nitrate-reductase enzymatic activity per se (i.e. independent of nitrate levels) is a variable and may account for any individual to individual variation. Here, we describe a minimally invasive protocol that allows for NR activity determination from human, rat and mouse tongue scrapes/swabs. We validate this method using selective application of antiseptic agents to the distal tongue surface which decreased NR activity by >80% and show that bacterial number is a significant variable in measured NR activities between males and females. Also, we show that NR activity is >80% lower in smokers (humans) and after bromine gas exposure (mice), suggesting that exposure to inhaled reactive substances inhibit NR activity identifying a potentially new mechanism by which environmental toxicants promote dysfunction in NO-bioavailability. The described method will facilitate studies testing whether NR specific activity is a variable in different pathophysiologic settings, and in turn how this activity modulates enterosalivary nitrate-reduction.
Keywords: Antiseptic; Chlorohexidine; Halogen; Microbiome; Nitric oxide; Smoking.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
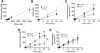
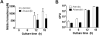
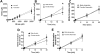


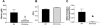
References
-
- Lundberg JO, Gladwin MT, Ahluwalia A, Benjamin N, Bryan NS, Butler A, Cabrales P, Fago A, Feelisch M, Ford PC, Freeman BA, Frenneaux M, Friedman J, Kelm M, Kevil CG, Kim-Shapiro DB, Kozlov AV, Lancaster JR, Jr, Lefer DJ, McColl K, McCurry K, Patel RP, Petersson J, Rassaf T, Reutov VP, Richter-Addo GB, Schechter A, Shiva S, Tsuchiya K, van Faassen EE, Webb AJ, Zuckerbraun BS, Zweier JL, Weitzberg E. Nitrate and nitrite in biology, nutrition and therapeutics. Nat Chem Biol. 2009;5:865–869. - PMC - PubMed
-
- Lundberg JO, Weitzberg E, Cole JA, Benjamin N. Nitrate, bacteria and human health. Nat Rev Microbiol. 2004;2:593–602. - PubMed
-
- Doel JJ, Benjamin N, Hector MP, Rogers M, Allaker RP. Evaluation of bacterial nitrate reduction in the human oral cavity. Eur J Oral Sci. 2005;113:14–19. - PubMed
-
- Lundberg JO, Gladwin MT, Weitzberg E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat Rev Drug Discov. 2015;14:623–641. - PubMed
-
- Hezel MP, Weitzberg E. The oral microbiome and nitric oxide homoeostasis. Oral Dis. 2015;21:7–16. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

