Apremilast, an oral phosphodiesterase 4 inhibitor, in the treatment of Japanese patients with moderate to severe plaque psoriasis: Efficacy, safety and tolerability results from a phase 2b randomized controlled trial
- PMID: 28391657
- PMCID: PMC5573969
- DOI: 10.1111/1346-8138.13829
Apremilast, an oral phosphodiesterase 4 inhibitor, in the treatment of Japanese patients with moderate to severe plaque psoriasis: Efficacy, safety and tolerability results from a phase 2b randomized controlled trial
Abstract
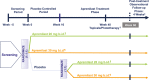
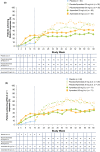
Apremilast, an oral, small-molecule phosphodiesterase 4 inhibitor, works intracellularly within immune cells to regulate inflammatory mediators. This phase 2b randomized, placebo-controlled study evaluated efficacy and safety of apremilast among Japanese patients with moderate to severe plaque psoriasis. In total, 254 patients were randomized to placebo, apremilast 20 mg b.i.d. (apremilast 20) or apremilast 30 mg b.i.d. (apremilast 30) through week 16; thereafter, all placebo patients were re-randomized to apremilast 20 or 30 through week 68. Efficacy assessments included achievement of 75% or more reduction from baseline in Psoriasis Area and Severity Index score (PASI-75; primary) and achievement of static Physician Global Assessment (sPGA; secondary) score of 0 (clear) or 1 (minimal) at week 16. Safety was assessed through week 68. At week 16, PASI-75 response rates were 7.1% (placebo), 23.5% (apremilast 20; P = 0.0032 vs placebo) and 28.2% (apremilast 30; P = 0.0003 vs placebo); sPGA response rates (score of 0 or 1) were 8.8% (placebo), 23.9% (apremilast 20; P = 0.0165 vs placebo) and 29.6% (apremilast 30; P = 0.0020 vs placebo). Responses were maintained with apremilast through week 68. Most common adverse events (AEs) with placebo, apremilast 20 and apremilast 30 (0-16 weeks) were nasopharyngitis (8.3%, 11.8%, 11.8%), diarrhea (1.2%, 8.2%, 9.4%), and abdominal discomfort (1.2%, 1.2%, 7.1%), respectively. Exposure-adjusted incidence of these AEs did not increase with continued apremilast treatment (up to 68 weeks). Apremilast demonstrated efficacy and safety in Japanese patients with moderate to severe plaque psoriasis through 68 weeks that was generally consistent with prior studies.
Keywords: Psoriasis Area and Severity Index; apremilast; phosphodiesterase 4; phosphodiesterase 4 inhibitor; psoriasis.
© 2017 The Authors. The Journal of Dermatology published by John Wiley & Sons Australia, Ltd on behalf of Japanese Dermatological Association.
Figures


References
-
- Parisi R, Symmons DP, Griffiths CE, Ashcroft DM. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol 2013; 133: 377–385. - PubMed
-
- Hsu S, Papp KA, Lebwohl MG et al Consensus guidelines for the management of plaque psoriasis. Arch Dermatol 2012; 148: 95–102. - PubMed
-
- Lebwohl MG, Bachelez H, Barker J et al Patient perspectives in the management of psoriasis: results from the population‐based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol 2014; 70: 871–881. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

