A Descending Neuron Correlated with the Rapid Steering Maneuvers of Flying Drosophila
- PMID: 28392112
- PMCID: PMC6309624
- DOI: 10.1016/j.cub.2017.03.004
A Descending Neuron Correlated with the Rapid Steering Maneuvers of Flying Drosophila
Abstract
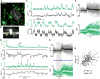
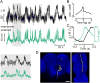
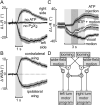
To navigate through the world, animals must stabilize their path against disturbances and change direction to avoid obstacles and to search for resources [1, 2]. Locomotion is thus guided by sensory cues but also depends on intrinsic processes, such as motivation and physiological state. Flies, for example, turn with the direction of large-field rotatory motion, an optomotor reflex that is thought to help them fly straight [3-5]. Occasionally, however, they execute fast turns, called body saccades, either spontaneously or in response to patterns of visual motion such as expansion [6-8]. These turns can be measured in tethered flying Drosophila [3, 4, 9], which facilitates the study of underlying neural mechanisms. Whereas there is evidence for an efference copy input to visual interneurons during saccades [10], the circuits that control spontaneous and visually elicited saccades are not well known. Using two-photon calcium imaging and electrophysiological recordings in tethered flying Drosophila, we have identified a descending neuron whose activity is correlated with both spontaneous and visually elicited turns during tethered flight. The cell's activity in open- and closed-loop experiments suggests that it does not underlie slower compensatory responses to horizontal motion but rather controls rapid changes in flight path. The activity of this neuron can explain some of the behavioral variability observed in response to visual motion and appears sufficient for eliciting turns when artificially activated. This work provides an entry point into studying the circuits underlying the control of rapid steering maneuvers in the fly brain.
Keywords: Drosophila; descending neuron; flight; saccades; spontaneous behavior.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures
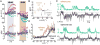
References
-
- Collett TS, Land MF. Visual control of flight behaviour in the hoverfly Syritta pipiens L. J Comp Physiol. 1975;1:1–66.
-
- Budick SA, O'Malley DM. Locomotor repertoire of the larval zebrafish: swimming, turning and prey capture. J Exp Biol. 2000;17:2565–2579. - PubMed
-
- Götz KG. Flight control in Drosophila by visual perception of motion. Kybernetik. 1968;6:199–208. - PubMed
-
- Tammero LF, Frye MA, Dickinson MH. Spatial organization of visuomotor reflexes in Drosophila. J Exp Biol. 2004;1:113–122. - PubMed
-
- Mronz M, Lehmann F. The free-flight response of Drosophila to motion of the visual environment. J Exp Biol. 2008;13:2026–2045. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

