Exploring the influence of indololactone structure on selectivity for binding to the C1 domains of PKCα, PKCε, and RasGRP
- PMID: 28392275
- PMCID: PMC5493039
- DOI: 10.1016/j.bmc.2017.03.022
Exploring the influence of indololactone structure on selectivity for binding to the C1 domains of PKCα, PKCε, and RasGRP
Abstract
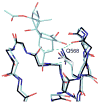
C1 domain-containing proteins, such as protein kinase C (PKC), have a central role in cellular signal transduction. Their involvement in many diseases, including cancer, cardiovascular disease, and immunological and neurological disorders has been extensively demonstrated and has prompted a search for small molecules to modulate their activity. By employing a diacylglycerol (DAG)-lactone template, we have been able to develop ultra potent analogs of diacylglycerol with nanomolar binding affinities approaching those of complex natural products such as phorbol esters and bryostatins. One current challenge is the development of selective ligands capable of discriminating between different protein family members. Recently, structure-activity relationship studies have shown that the introduction of an indole ring as a DAG-lactone substituent yielded selective Ras guanine nucleotide-releasing protein (RasGRP1) activators when compared to PKCα and PKCε. In the present work, we examine the effects of ligand selectivity relative to the orientation of the indole ring and the nature of the DAG-lactone template itself. Our results show that the indole ring must be attached to the lactone moiety through the sn-2 position in order to achieve RasGRP1 selectivity.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures
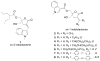
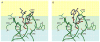
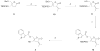

Similar articles
-
Synthesis, biological, and biophysical studies of DAG-indololactones designed as selective activators of RasGRP.Bioorg Med Chem. 2014 Jun 15;22(12):3123-40. doi: 10.1016/j.bmc.2014.04.024. Epub 2014 Apr 20. Bioorg Med Chem. 2014. PMID: 24794745 Free PMC article.
-
A novel diacylglycerol-lactone shows marked selectivity in vitro among C1 domains of protein kinase C (PKC) isoforms alpha and delta as well as selectivity for RasGRP compared with PKCalpha.J Biol Chem. 2005 Jul 22;280(29):27329-38. doi: 10.1074/jbc.M414132200. Epub 2005 May 27. J Biol Chem. 2005. PMID: 15923197
-
α-Arylidene Diacylglycerol-Lactones (DAG-Lactones) as Selective Ras Guanine-Releasing Protein 3 (RasGRP3) Ligands.J Med Chem. 2018 Jul 26;61(14):6261-6276. doi: 10.1021/acs.jmedchem.8b00661. Epub 2018 Jul 3. J Med Chem. 2018. PMID: 29860841
-
Indolactam and benzolactam compounds as new medicinal leads with binding selectivity for C1 domains of protein kinase C isozymes.Curr Pharm Des. 2004;10(12):1371-85. doi: 10.2174/1381612043384907. Curr Pharm Des. 2004. PMID: 15134488 Review.
-
Synthetic diacylglycerols (DAG) and DAG-lactones as activators of protein kinase C (PK-C).Acc Chem Res. 2003 Jun;36(6):434-43. doi: 10.1021/ar020124b. Acc Chem Res. 2003. PMID: 12809530 Review.
Cited by
-
Regulation of the Ras-Related Signaling Pathway by Small Molecules Containing an Indole Core Scaffold: A Potential Antitumor Therapy.Front Pharmacol. 2020 Mar 13;11:280. doi: 10.3389/fphar.2020.00280. eCollection 2020. Front Pharmacol. 2020. PMID: 32231571 Free PMC article. Review.
-
New insights into transcription elongation control of HIV-1 latency and rebound.Trends Immunol. 2023 Jan;44(1):60-71. doi: 10.1016/j.it.2022.11.003. Epub 2022 Dec 8. Trends Immunol. 2023. PMID: 36503686 Free PMC article. Review.
-
A Focused Review of Ras Guanine Nucleotide-Releasing Protein 1 in Immune Cells and Cancer.Int J Mol Sci. 2023 Jan 13;24(2):1652. doi: 10.3390/ijms24021652. Int J Mol Sci. 2023. PMID: 36675167 Free PMC article. Review.
-
Scaffold hopping from (5-hydroxymethyl) isophthalates to multisubstituted pyrimidines diminishes binding affinity to the C1 domain of protein kinase C.PLoS One. 2018 Apr 11;13(4):e0195668. doi: 10.1371/journal.pone.0195668. eCollection 2018. PLoS One. 2018. PMID: 29641588 Free PMC article.
-
The cell biology of HIV-1 latency and rebound.Retrovirology. 2024 Apr 5;21(1):6. doi: 10.1186/s12977-024-00639-w. Retrovirology. 2024. PMID: 38580979 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

