Modulation of experimental arthritis by vagal sensory and central brain stimulation
- PMID: 28392428
- PMCID: PMC6330674
- DOI: 10.1016/j.bbi.2017.04.003
Modulation of experimental arthritis by vagal sensory and central brain stimulation
Abstract
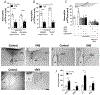
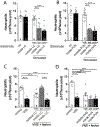
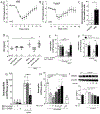
Articular inflammation is a major clinical burden in multiple inflammatory diseases, especially in rheumatoid arthritis. Biological anti-rheumatic drug therapies are expensive and increase the risk of systemic immunosuppression, infections, and malignancies. Here, we report that vagus nerve stimulation controls arthritic joint inflammation by inducing local regulation of innate immune response. Most of the previous studies of neuromodulation focused on vagal regulation of inflammation via the efferent peripheral pathway toward the viscera. Here, we report that vagal stimulation modulates arthritic joint inflammation through a novel "afferent" pathway mediated by the locus coeruleus (LC) of the central nervous system. Afferent vagal stimulation activates two sympatho-excitatory brain areas: the paraventricular hypothalamic nucleus (PVN) and the LC. The integrity of the LC, but not that of the PVN, is critical for vagal control of arthritic joint inflammation. Afferent vagal stimulation suppresses articular inflammation in the ipsilateral, but not in the contralateral knee to the hemispheric LC lesion. Central stimulation is followed by subsequent activation of joint sympathetic nerve terminals inducing articular norepinephrine release. Selective adrenergic beta-blockers prevent the effects of articular norepinephrine and thereby abrogate vagal control of arthritic joint inflammation. These results reveals a novel neuro-immune brain map with afferent vagal signals controlling side-specific articular inflammation through specific inflammatory-processing brain centers and joint sympathetic innervations.
Keywords: Arthritis; Neuroimmune interactions; Neuroimmunomodulation; Neutrophil migration; Sympathetic nervous system; Vagus nerve.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosures
The authors have no financial conflicts of interest.
Figures


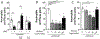

References
-
- Armour JA, Randall WC, 1975. Functional anatomy of canine cardiac nerves. Acta Anat. (Basel) 91, 510–528. - PubMed
-
- Bassi GS, Brognara F, Castania JA, Talbot J, Cunha TM, Cunha FQ, Ulloa L, Kanashiro A, Dias DP, Salgado HC, 2015. Baroreflex activation in conscious rats modulates the joint inflammatory response via sympathetic function. Brain Behav. Immun 49, 140–147. 10.1016/j.bbi.2015.05.002. - DOI - PMC - PubMed
-
- Bellinger DL, Nance DM, Lorton D, 2013. Innervation of the Immune System In: The Wiley-Blackwell Handbook of Psychoneuroimmunology. Wiley-Blackwell, pp. 24–72.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

