Therapeutic gene editing: delivery and regulatory perspectives
- PMID: 28392568
- PMCID: PMC5520188
- DOI: 10.1038/aps.2017.2
Therapeutic gene editing: delivery and regulatory perspectives
Abstract
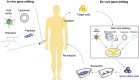
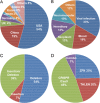
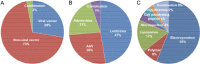
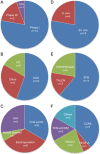
Gene-editing technology is an emerging therapeutic modality for manipulating the eukaryotic genome by using target-sequence-specific engineered nucleases. Because of the exceptional advantages that gene-editing technology offers in facilitating the accurate correction of sequences in a genome, gene editing-based therapy is being aggressively developed as a next-generation therapeutic approach to treat a wide range of diseases. However, strategies for precise engineering and delivery of gene-editing nucleases, including zinc finger nucleases, transcription activator-like effector nuclease, and CRISPR/Cas9 (clustered regularly interspaced short palindromic repeats-associated nuclease Cas9), present major obstacles to the development of gene-editing therapies, as with other gene-targeting therapeutics. Currently, viral and non-viral vectors are being studied for the delivery of these nucleases into cells in the form of DNA, mRNA, or proteins. Clinical trials are already ongoing, and in vivo studies are actively investigating the applicability of CRISPR/Cas9 techniques. However, the concept of correcting the genome poses major concerns from a regulatory perspective, especially in terms of safety. This review addresses current research trends and delivery strategies for gene editing-based therapeutics in non-clinical and clinical settings and considers the associated regulatory issues.
Figures

Similar articles
-
CRISPR/Cas9 technology as a potent molecular tool for gene therapy.J Cell Physiol. 2019 Aug;234(8):12267-12277. doi: 10.1002/jcp.27972. Epub 2019 Jan 30. J Cell Physiol. 2019. PMID: 30697727 Review.
-
Non-viral delivery of genome-editing nucleases for gene therapy.Gene Ther. 2017 Mar;24(3):144-150. doi: 10.1038/gt.2016.72. Epub 2016 Oct 31. Gene Ther. 2017. PMID: 27797355 Review.
-
Gene targeting technologies in rats: zinc finger nucleases, transcription activator-like effector nucleases, and clustered regularly interspaced short palindromic repeats.Dev Growth Differ. 2014 Jan;56(1):46-52. doi: 10.1111/dgd.12110. Epub 2013 Dec 27. Dev Growth Differ. 2014. PMID: 24372523 Review.
-
Therapeutic Genome Editing and In Vivo Delivery.AAPS J. 2021 Jun 2;23(4):80. doi: 10.1208/s12248-021-00613-w. AAPS J. 2021. PMID: 34080099 Review.
-
[CRISPR/Cas9 technology in disease research and therapy: a review].Sheng Wu Gong Cheng Xue Bao. 2021 Apr 25;37(4):1205-1228. doi: 10.13345/j.cjb.200401. Sheng Wu Gong Cheng Xue Bao. 2021. PMID: 33973436 Review. Chinese.
Cited by
-
Update on clinical gene therapy for hemophilia.Blood. 2019 Jan 31;133(5):407-414. doi: 10.1182/blood-2018-07-820720. Epub 2018 Dec 17. Blood. 2019. PMID: 30559260 Free PMC article. Review.
-
Current Advancements in Transdermal Biosensing and Targeted Drug Delivery.Sensors (Basel). 2019 Feb 28;19(5):1028. doi: 10.3390/s19051028. Sensors (Basel). 2019. PMID: 30823435 Free PMC article. Review.
-
How Far Are Non-Viral Vectors to Come of Age and Reach Clinical Translation in Gene Therapy?Int J Mol Sci. 2021 Jul 14;22(14):7545. doi: 10.3390/ijms22147545. Int J Mol Sci. 2021. PMID: 34299164 Free PMC article. Review.
-
Innovative approaches in stem cell therapy: revolutionizing cancer treatment and advancing neurobiology - a comprehensive review.Int J Surg. 2024 Dec 1;110(12):7528-7545. doi: 10.1097/JS9.0000000000002111. Int J Surg. 2024. PMID: 39377430 Free PMC article. Review.
-
Modulation of DNA double-strand break repair as a strategy to improve precise genome editing.Oncogene. 2020 Oct;39(41):6393-6405. doi: 10.1038/s41388-020-01445-2. Epub 2020 Sep 3. Oncogene. 2020. PMID: 32884115 Review.
References
-
- Urnov FD, Rebar EJ, Holmes MC, Zhang HS, Gregory PD. Genome editing with engineered zinc finger nucleases. Nat Rev Genet 2010; 11: 636–46. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

