Evaluation of zinc-doped mesoporous hydroxyapatite microspheres for the construction of a novel biomimetic scaffold optimized for bone augmentation
- PMID: 28392688
- PMCID: PMC5373825
- DOI: 10.2147/IJN.S126505
Evaluation of zinc-doped mesoporous hydroxyapatite microspheres for the construction of a novel biomimetic scaffold optimized for bone augmentation
Abstract
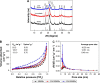
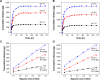
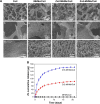
Biomaterials with high osteogenic activity are desirable for sufficient healing of bone defects resulting from trauma, tumor, infection, and congenital abnormalities. Synthetic materials mimicking the structure and composition of human trabecular bone are of considerable potential in bone augmentation. In the present study, a zinc (Zn)-doped mesoporous hydroxyapatite microspheres (Zn-MHMs)/collagen scaffold (Zn-MHMs/Coll) was developed through a lyophilization fabrication process and designed to mimic the trabecular bone. The Zn-MHMs were synthesized through a microwave-hydrothermal method by using creatine phosphate as an organic phosphorus source. Zn-MHMs that consist of hydroxyapatite nanosheets showed relatively uniform spherical morphology, mesoporous hollow structure, high specific surface area, and homogeneous Zn distribution. They were additionally investigated as a drug nanocarrier, which was efficient in drug delivery and presented a pH-responsive drug release behavior. Furthermore, they were incorporated into the collagen matrix to construct a biomimetic scaffold optimized for bone tissue regeneration. The Zn-MHMs/Coll scaffolds showed an interconnected pore structure in the range of 100-300 μm and a sustained release of Zn ions. More importantly, the Zn-MHMs/Coll scaffolds could enhance the osteogenic differentiation of rat bone marrow-derived mesenchymal stem cells. Finally, the bone defect repair results of critical-sized femoral condyle defect rat model demonstrated that the Zn-MHMs/Coll scaffolds could enhance bone regeneration compared with the Coll or MHMs/Coll scaffolds. The results suggest that the biomimetic Zn-MHMs/Coll scaffolds may be of enormous potential in bone repair and regeneration.
Keywords: biomimicry; bone regeneration; drug delivery; mesoporous hydroxyapatite microspheres; scaffold; zinc.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures





Similar articles
-
Enhanced osteogenesis and angiogenesis by mesoporous hydroxyapatite microspheres-derived simvastatin sustained release system for superior bone regeneration.Sci Rep. 2017 Mar 13;7:44129. doi: 10.1038/srep44129. Sci Rep. 2017. PMID: 28287178 Free PMC article.
-
Copper-doped mesoporous hydroxyapatite microspheres synthesized by a microwave-hydrothermal method using creatine phosphate as an organic phosphorus source: application in drug delivery and enhanced bone regeneration.J Mater Chem B. 2017 Feb 7;5(5):1039-1052. doi: 10.1039/c6tb02747d. Epub 2017 Jan 18. J Mater Chem B. 2017. PMID: 32263882
-
Strontium-Doped Amorphous Calcium Phosphate Porous Microspheres Synthesized through a Microwave-Hydrothermal Method Using Fructose 1,6-Bisphosphate as an Organic Phosphorus Source: Application in Drug Delivery and Enhanced Bone Regeneration.ACS Appl Mater Interfaces. 2017 Feb 1;9(4):3306-3317. doi: 10.1021/acsami.6b12325. Epub 2017 Jan 24. ACS Appl Mater Interfaces. 2017. PMID: 28068758
-
Nanofibrous Microspheres: A Biomimetic Platform for Bone Tissue Regeneration.ACS Appl Bio Mater. 2024 Jul 15;7(7):4270-4292. doi: 10.1021/acsabm.4c00613. Epub 2024 Jul 1. ACS Appl Bio Mater. 2024. PMID: 38950103 Free PMC article. Review.
-
Biomimetic Mineralized Collagen Scaffolds for Bone Tissue Engineering: Strategies on Elaborate Fabrication for Bioactivity Improvement.Small. 2025 Jan;21(3):e2406441. doi: 10.1002/smll.202406441. Epub 2024 Nov 24. Small. 2025. PMID: 39580700 Review.
Cited by
-
Study on osteogenesis of zinc-loaded carbon nanotubes/chitosan composite biomaterials in rat skull defects.J Mater Sci Mater Med. 2020 Jan 21;31(2):15. doi: 10.1007/s10856-019-6338-3. J Mater Sci Mater Med. 2020. PMID: 31965348
-
Sustained zinc release in cooperation with CaP scaffold promoted bone regeneration via directing stem cell fate and triggering a pro-healing immune stimuli.J Nanobiotechnology. 2021 Jul 12;19(1):207. doi: 10.1186/s12951-021-00956-8. J Nanobiotechnology. 2021. PMID: 34247649 Free PMC article.
-
Composite or Modified Hydroxyapatite Microspheres as Drug Delivery Carrier for Bone and Tooth Tissue Engineering.Curr Med Chem. 2025;32(5):974-981. doi: 10.2174/0109298673303632240320073606. Curr Med Chem. 2025. PMID: 38523515 Review.
-
Peptoid-Cross-Linked Hydrogel Stiffness Modulates Human Mesenchymal Stromal Cell Immunoregulatory Potential in the Presence of Interferon-Gamma.Macromol Biosci. 2024 Jul;24(7):e2400111. doi: 10.1002/mabi.202400111. Epub 2024 Apr 18. Macromol Biosci. 2024. PMID: 38567626 Free PMC article.
-
Calcium Phosphate Delivery Systems for Regeneration and Biomineralization of Mineralized Tissues of the Craniofacial Complex.Mol Pharm. 2023 Feb 6;20(2):810-828. doi: 10.1021/acs.molpharmaceut.2c00652. Epub 2023 Jan 18. Mol Pharm. 2023. PMID: 36652561 Free PMC article. Review.
References
-
- Vallet-Regi M, Ruiz-Hernandez E. Bioceramics: from bone regeneration to cancer nanomedicine. Adv Mater. 2011;23(44):5177–5218. - PubMed
-
- Habibovic P, Barralet JE. Bioinorganics and biomaterials: bone repair. Acta Biomater. 2011;7(8):3013–3026. - PubMed
-
- Bucholz RW. Nonallograft osteoconductive bone graft substitutes. Clin Orthop Relat Res. 2002;(395):44–52. - PubMed
-
- Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res. 1996;(329):300–309. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

