Hyaluronic acid-nimesulide conjugates as anticancer drugs against CD44-overexpressing HT-29 colorectal cancer in vitro and in vivo
- PMID: 28392690
- PMCID: PMC5376212
- DOI: 10.2147/IJN.S120847
Hyaluronic acid-nimesulide conjugates as anticancer drugs against CD44-overexpressing HT-29 colorectal cancer in vitro and in vivo
Abstract
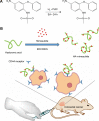
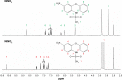
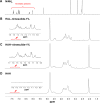
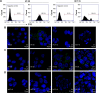
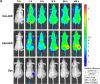
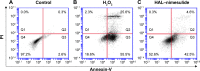
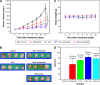
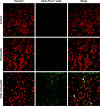
Carrier-mediated drug delivery systems are promising therapeutics for targeted delivery and improved efficacy and safety of potent cytotoxic drugs. Nimesulide is a multifactorial cyclooxygenase 2 nonsteroidal anti-inflammatory drug with analgesic, antipyretic and potent anticancer properties; however, the low solubility of nimesulide limits its applications. Drugs conjugated with hyaluronic acid (HA) are innovative carrier-mediated drug delivery systems characterized by CD44-mediated endocytosis of HA and intracellular drug release. In this study, hydrophobic nimesulide was conjugated to HA of two different molecular weights (360 kDa as HA with high molecular weight [HAH] and 43kDa as HA with low molecular weight [HAL]) to improve its tumor-targeting ability and hydrophilicity. Our results showed that hydrogenated nimesulide (N-[4-amino-2-phenoxyphenyl]methanesulfonamide) was successfully conjugated with both HA types by carbodiimide coupling and the degree of substitution of nimesulide was 1%, which was characterized by 1H nuclear magnetic resonance 400 MHz and total correlation spectroscopy. Both Alexa Fluor® 647 labeled HAH and HAL could selectively accumulate in CD44-overexpressing HT-29 colorectal tumor area in vivo, as observed by in vivo imaging system. In the in vitro cytotoxic test, HA-nimesulide conjugate displayed >46% cell killing ability at a nimesulide concentration of 400 µM in HT-29 cells, whereas exiguous cytotoxic effects were observed on HCT-15 cells, indicating that HA-nimesulide causes cell death in CD44-overexpressing HT-29 cells. Regarding in vivo antitumor study, both HAL-nimesulide and HAH-nimesulide caused rapid tumor shrinkage within 3 days and successfully inhibited tumor growth, which reached 82.3% and 76.4% at day 24 through apoptotic mechanism in HT-29 xenografted mice, without noticeable morphologic differences in the liver or kidney, respectively. These results indicated that HA-nimesulide with improved selectivity through HA/CD44 receptor interactions has the potential to enhance the therapeutic efficacy and safety of nimesulide for cancer treatment.
Keywords: CD44; COX-2 inhibitor; colorectal cancer; hyaluronic acid; nimesulide.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures




Similar articles
-
Hyaluronic acid-shelled acid-activatable paclitaxel prodrug micelles effectively target and treat CD44-overexpressing human breast tumor xenografts in vivo.Biomaterials. 2016 Apr;84:250-261. doi: 10.1016/j.biomaterials.2016.01.049. Epub 2016 Jan 23. Biomaterials. 2016. PMID: 26851390
-
Reduction-sensitive CD44 receptor-targeted hyaluronic acid derivative micelles for doxorubicin delivery.Int J Nanomedicine. 2018 Jul 26;13:4361-4378. doi: 10.2147/IJN.S165359. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30100720 Free PMC article.
-
Smart nanoparticles with a detachable outer shell for maximized synergistic antitumor efficacy of therapeutics with varying physicochemical properties.J Control Release. 2016 Dec 10;243:54-68. doi: 10.1016/j.jconrel.2016.09.036. Epub 2016 Oct 1. J Control Release. 2016. PMID: 27702595
-
Advances in Hyaluronic Acid-Based Drug Delivery Systems.Curr Drug Targets. 2016;17(6):720-30. doi: 10.2174/1389450116666150531155200. Curr Drug Targets. 2016. PMID: 26028046 Review.
-
Anticancer therapeutics: targeting macromolecules and nanocarriers to hyaluronan or CD44, a hyaluronan receptor.Mol Pharm. 2008 Jul-Aug;5(4):474-86. doi: 10.1021/mp800024g. Epub 2008 Jun 3. Mol Pharm. 2008. PMID: 18547053 Free PMC article. Review.
Cited by
-
Rational Design of Polyglutamic Acid Delivering an Optimized Combination of Drugs Targeting Mutated BRAF and MEK in Melanoma.Adv Ther (Weinh). 2020 Aug;3(8):2000028. doi: 10.1002/adtp.202000028. Epub 2020 May 12. Adv Ther (Weinh). 2020. PMID: 35754977 Free PMC article.
-
Synthesis and Different Effects of Biotinylated PAMAM G3 Dendrimer Substituted with Nimesulide in Human Normal Fibroblasts and Squamous Carcinoma Cells.Biomolecules. 2019 Sep 1;9(9):437. doi: 10.3390/biom9090437. Biomolecules. 2019. PMID: 31480608 Free PMC article.
-
BH3 profiling discriminates the anti‑apoptotic status of 5‑fluorouracil‑resistant colon cancer cells.Oncol Rep. 2019 Dec;42(6):2416-2425. doi: 10.3892/or.2019.7373. Epub 2019 Oct 15. Oncol Rep. 2019. PMID: 31638265 Free PMC article.
-
Hyaluronic Acid Capped, Irinotecan and Gene Co-Loaded Lipid-Polymer Hybrid Nanocarrier-Based Combination Therapy Platform for Colorectal Cancer.Drug Des Devel Ther. 2020 Mar 12;14:1095-1105. doi: 10.2147/DDDT.S230306. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 32210538 Free PMC article.
-
A Glucuronic Acid-Palmitoylethanolamide Conjugate (GLUPEA) Is an Innovative Drug Delivery System and a Potential Bioregulator.Cells. 2021 Feb 20;10(2):450. doi: 10.3390/cells10020450. Cells. 2021. PMID: 33672574 Free PMC article.
References
-
- Chang YT, Tseng HC, Huang CC, et al. Relative down-regulation of apoptosis and autophagy genes in colorectal cancer. Eur J Clin Invest. 2011;41(1):84–92. - PubMed
-
- André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350(23):2343–2351. - PubMed
-
- Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355(9209):1041–1047. - PubMed
-
- Li J, Hou N, Faried A, Tsutsumi S, Kuwano H. Inhibition of autophagy augments 5-fluorouracil chemotherapy in human colon cancer in vitro and in vivo model. Eur J Cancer. 2010;46(10):1900–1909. - PubMed
-
- Longley DB, Allen WL, Johnston PG. Drug resistance, predictive markers and pharmacogenomics in colorectal cancer. Biochim Biophys Acta. 2006;1766(2):184–196. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

