Demystifying EQA statistics and reports
- PMID: 28392725
- PMCID: PMC5382862
- DOI: 10.11613/BM.2017.006
Demystifying EQA statistics and reports
Abstract
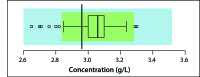
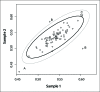
Reports act as an important feedback tool in External Quality Assessment (EQA). Their main role is to score laboratories for their performance in an EQA round. The most common scores that apply to quantitative data are Q- and Z-scores. To calculate these scores, EQA providers need to have an assigned value and standard deviation for the sample. Both assigned values and standard deviations can be derived chemically or statistically. When derived statistically, different anomalies against the normal distribution of the data have to be handled. Various procedures for evaluating laboratories are able to handle these anomalies. Formal tests and graphical representation techniques are discussed and suggestions are given to help choosing between the different evaluations techniques. In order to obtain reliable estimates for calculating performance scores, a satisfactory number of data is needed. There is no general agreement about the minimal number that is needed. A solution for very small numbers is proposed by changing the limits of evaluation. Apart from analyte- and sample-specific laboratory evaluation, supplementary information can be obtained by combining results for different analytes and samples. Various techniques are overviewed. It is shown that combining results leads to supplementary information, not only for quantitative, but also for qualitative and semi-quantitative analytes.
Keywords: Q-score; Z-score; external quality assessment; statistics.
Conflict of interest statement
None declared.
Figures

References
-
- Hill P, Uldall A, Wilding P. Fundamentals for external quality assessment (EQA). Available at: http://www.ifcc.org/ifccfiles/docs/fundamentals-for-eqa.pdf. Accessed February 12th 2016.
-
- Wong SK. Evaluation of the use of consensus values in proficiency testing programmes. Accredit Qual Assur. 2005;10:409–14. 10.1007/s00769-005-0029-0 - DOI
-
- Visser RG. Interpretation of interlaboratory comparison results to evaluate laboratory proficiency. Accredit Qual Assur. 2006;10:521–6. 10.1007/s00769-005-0051-2 - DOI
-
- Rosario P, Martínez JL, Silván JM. Comparison of different statistical methods for evaluation of proficiency test data. Accredit Qual Assur. 2008;13:493–9. 10.1007/s00769-008-0413-7 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
