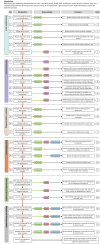
Interpretation of EQA results and EQA-based trouble shooting
- PMID: 28392726
- PMCID: PMC5382861
- DOI: 10.11613/BM.2017.007
Interpretation of EQA results and EQA-based trouble shooting
Abstract
Important objectives of External Quality Assessment (EQA) is to detect analytical errors and make corrective actions. The aim of this paper is to describe knowledge required to interpret EQA results and present a structured approach on how to handle deviating EQA results. The value of EQA and how the EQA result should be interpreted depends on five key points: the control material, the target value, the number of replicates, the acceptance limits and between lot variations in reagents used in measurement procedures. This will also affect the process of finding the sources of errors when they appear. The ideal EQA sample has two important properties: it behaves as a native patient sample in all methods (is commutable) and has a target value established with a reference method. If either of these two criteria is not entirely fulfilled, results not related to the performance of the laboratory may arise. To help and guide the laboratories in handling a deviating EQA result, the Norwegian Clinical Chemistry EQA Program (NKK) has developed a flowchart with additional comments that could be used by the laboratories e.g. in their quality system, to document action against deviations in EQA. This EQA-based trouble-shooting tool has been developed further in cooperation with the External quality Control for Assays and Tests (ECAT) Foundation. This flowchart will become available in a public domain, i.e. the website of the European organisation for External Quality Assurance Providers in Laboratory Medicine (EQALM).
Keywords: EQA error; between lot variation; commutability, reference method; external quality assessment.
Conflict of interest statement
None declared.
Figures
References
-
- Belk WP, Sunderman FW. A survey of the accuracy of chemical analyses in clinical laboratories. Am J Clin Pathol. 1947;17:853–96. https://doi.org/10.1093/ajcp/17.11.853 10.1093/ajcp/17.11.853 - DOI - DOI - PubMed
-
- Libeer JC, Baadenhuijsen H, Fraser CG, Petersen PH, Ricos C, Stockl D, et al. Characterization and classification of external quality assessment schemes (EQA) according to objectives such as evaluation of method and participant bias and standard deviation. External Quality Assessment (EQA) Working Group A on Analytical Goals in Laboratory Medicine. Eur J Clin Chem Clin Biochem. 1996;34:665–78. - PubMed
-
- International Organization for Standardization. ISO 15189: medical laboratories: particular requirements for quality and competence. Geneva, Switzerland: International Organization for Standardization; 2012.
-
- Miller WG, Jones GR, Horowitz GL, Weykamp C. Proficiency testing/external quality assessment: current challenges and future directions. Clin Chem. 2011;57:1670–80. https://doi.org/10.1373/clinchem.2011.168641 10.1373/clinchem.2011.168641 - DOI - DOI - PubMed
-
- Kristensen GB, Christensen NG, Thue G, Sandberg S. Between-lot variation in external quality assessment of glucose: clinical importance and effect on participant performance evaluation. Clin Chem. 2005;51:1632–6. https://doi.org/10.1373/clinchem.2005.049080 10.1373/clinchem.2005.049080 - DOI - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical