Shared genetic influences between dimensional ASD and ADHD symptoms during child and adolescent development
- PMID: 28392908
- PMCID: PMC5379648
- DOI: 10.1186/s13229-017-0131-2
Shared genetic influences between dimensional ASD and ADHD symptoms during child and adolescent development
Abstract
Background: Shared genetic influences between attention-deficit/hyperactivity disorder (ADHD) symptoms and autism spectrum disorder (ASD) symptoms have been reported. Cross-trait genetic relationships are, however, subject to dynamic changes during development. We investigated the continuity of genetic overlap between ASD and ADHD symptoms in a general population sample during childhood and adolescence. We also studied uni- and cross-dimensional trait-disorder links with respect to genetic ADHD and ASD risk.
Methods: Social-communication difficulties (N ≤ 5551, Social and Communication Disorders Checklist, SCDC) and combined hyperactive-impulsive/inattentive ADHD symptoms (N ≤ 5678, Strengths and Difficulties Questionnaire, SDQ-ADHD) were repeatedly measured in a UK birth cohort (ALSPAC, age 7 to 17 years). Genome-wide summary statistics on clinical ASD (5305 cases; 5305 pseudo-controls) and ADHD (4163 cases; 12,040 controls/pseudo-controls) were available from the Psychiatric Genomics Consortium. Genetic trait variances and genetic overlap between phenotypes were estimated using genome-wide data.
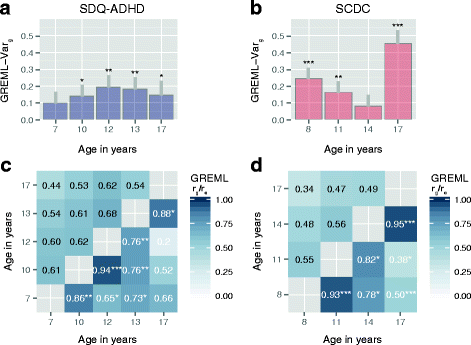
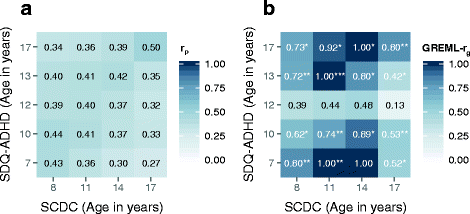
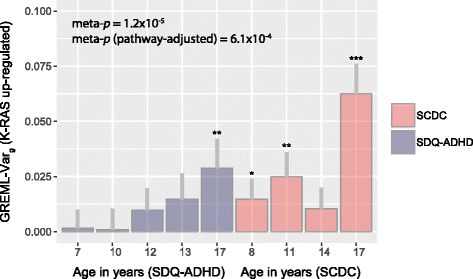
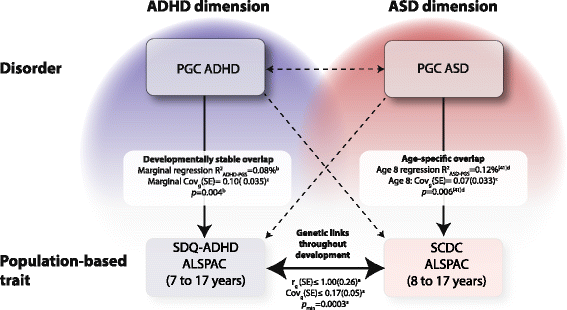
Results: In the general population, genetic influences for SCDC and SDQ-ADHD scores were shared throughout development. Genetic correlations across traits reached a similar strength and magnitude (cross-trait rg ≤ 1, pmin = 3 × 10-4) as those between repeated measures of the same trait (within-trait rg ≤ 0.94, pmin = 7 × 10-4). Shared genetic influences between traits, especially during later adolescence, may implicate variants in K-RAS signalling upregulated genes (p-meta = 6.4 × 10-4). Uni-dimensionally, each population-based trait mapped to the expected behavioural continuum: risk-increasing alleles for clinical ADHD were persistently associated with SDQ-ADHD scores throughout development (marginal regression R2 = 0.084%). An age-specific genetic overlap between clinical ASD and social-communication difficulties during childhood was also shown, as per previous reports. Cross-dimensionally, however, neither SCDC nor SDQ-ADHD scores were linked to genetic risk for disorder.
Conclusions: In the general population, genetic aetiologies between social-communication difficulties and ADHD symptoms are shared throughout child and adolescent development and may implicate similar biological pathways that co-vary during development. Within both the ASD and the ADHD dimension, population-based traits are also linked to clinical disorder, although much larger clinical discovery samples are required to reliably detect cross-dimensional trait-disorder relationships.
Keywords: ADHD symptoms; ALSPAC; Clinical ADHD; Genetic overlap; Social communication.
Figures
References
-
- Mulligan A, Anney RJL, O’Regan M, Chen W, Butler L, Fitzgerald M, et al. Autism symptoms in attention-deficit/hyperactivity disorder: a familial trait which correlates with conduct, oppositional defiant, language and motor disorders. J Autism Dev Disord. 2008;39:197–209. doi: 10.1007/s10803-008-0621-3. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

