Identification of 4-(3-Pyridyl)-4-oxobutyl-2'-deoxycytidine Adducts Formed in the Reaction of DNA with 4-(Acetoxymethylnitrosamino)-1-(3-pyridyl)-1-butanone: A Chemically Activated Form of Tobacco-Specific Carcinogens
- PMID: 28393135
- PMCID: PMC5377278
- DOI: 10.1021/acsomega.7b00072
Identification of 4-(3-Pyridyl)-4-oxobutyl-2'-deoxycytidine Adducts Formed in the Reaction of DNA with 4-(Acetoxymethylnitrosamino)-1-(3-pyridyl)-1-butanone: A Chemically Activated Form of Tobacco-Specific Carcinogens
Abstract
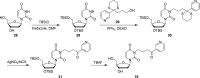
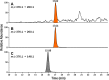
Metabolic activation of the carcinogenic tobacco-specific nitrosamines 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK, 1) and N'-nitrosonornicotine (NNN, 2) results in the formation of 4-(3-pyridyl)-4-oxobutyl (POB)-DNA adducts, several of which have been previously identified both in vitro and in tissues of laboratory animals treated with NNK or NNN. However, 2'-deoxycytidine adducts formed in this process have been incompletely examined in previous studies. Therefore, in this study we prepared characterized standards for the identification of previously unknown 2'-deoxycytidine and 2'-deoxyuridine adducts that could be produced in these reactions. The formation of these products in reactions of 4-(acetoxymethylnitrosamino)-1-(3-pyridyl)-1-butanone (NNKOAc, 3), a model 4-(3-pyridyl)-4-oxobutylating agent, with DNA was investigated. The major 2'-deoxycytidine adduct, identified as its stable cytosine analogue O2-[4-(3-pyridyl)-4-oxobut-1-yl]-cytosine (12), was O2-[4-(3-pyridyl)-4-oxobut-1-yl]-2'-deoxycytidine (13), whereas lesser amounts of 3-[4-(3-pyridyl)-4-oxobut-1-yl]-2'-deoxycytidine (14) and N4-[4-(3-pyridyl)-4-oxobut-1-yl]-2'-deoxycytidine (15) were also observed. The potential conversion of relatively unstable 2'-deoxycytidine adducts to stable 2'-deoxyuridine adducts by treatment of the adducted DNA with bisulfite was also investigated, but the harsh conditions associated with this approach prevented quantitation. The results of this study provide new validated standards for the study of 4-(3-pyridyl)-4-oxobutylation of DNA, a critical reaction in the carcinogenesis by 1 and 2, and demonstrate the presence of previously unidentified 2'-deoxycytidine adducts in this DNA.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





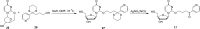

Similar articles
-
Quantitation of pyridyloxobutyl DNA adducts of tobacco-specific nitrosamines in rat tissue DNA by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry.Chem Res Toxicol. 2006 May;19(5):674-82. doi: 10.1021/tx050351x. Chem Res Toxicol. 2006. PMID: 16696570 Free PMC article.
-
Mass spectrometric analysis of relative levels of pyridyloxobutylation adducts formed in the reaction of DNA with a chemically activated form of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone.Chem Res Toxicol. 2005 Jun;18(6):1048-55. doi: 10.1021/tx050028u. Chem Res Toxicol. 2005. PMID: 15962940
-
Identification of adducts formed by pyridyloxobutylation of deoxyguanosine and DNA by 4-(acetoxymethylnitrosamino)-1-(3-pyridyl)-1-butanone, a chemically activated form of tobacco specific carcinogens.Chem Res Toxicol. 2003 May;16(5):616-26. doi: 10.1021/tx034003b. Chem Res Toxicol. 2003. PMID: 12755591
-
DNA adduct formation from tobacco-specific N-nitrosamines.Mutat Res. 1999 Mar 8;424(1-2):127-42. doi: 10.1016/s0027-5107(99)00014-7. Mutat Res. 1999. PMID: 10064856 Review.
-
Biomarkers for human uptake and metabolic activation of tobacco-specific nitrosamines.Cancer Res. 1994 Apr 1;54(7 Suppl):1912s-1917s. Cancer Res. 1994. PMID: 8137311 Review.
Cited by
-
Methyl DNA Phosphate Adduct Formation in Rats Treated Chronically with 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone and Enantiomers of Its Metabolite 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanol.Chem Res Toxicol. 2018 Jan 16;31(1):48-57. doi: 10.1021/acs.chemrestox.7b00281. Epub 2017 Nov 30. Chem Res Toxicol. 2018. PMID: 29131934 Free PMC article.
-
A Novel Adductomics Workflow Incorporating FeatureHunter Software: Rapid Detection of Nucleic Acid Modifications for Studying the Exposome.Environ Sci Technol. 2024 Jan 9;58(1):75-89. doi: 10.1021/acs.est.3c04674. Epub 2023 Dec 28. Environ Sci Technol. 2024. PMID: 38153287 Free PMC article.
-
Recent Studies on DNA Adducts Resulting from Human Exposure to Tobacco Smoke.Toxics. 2019 Mar 19;7(1):16. doi: 10.3390/toxics7010016. Toxics. 2019. PMID: 30893918 Free PMC article. Review.
-
Metabolism and DNA Adduct Formation of Tobacco-Specific N-Nitrosamines.Int J Mol Sci. 2022 May 4;23(9):5109. doi: 10.3390/ijms23095109. Int J Mol Sci. 2022. PMID: 35563500 Free PMC article. Review.
-
Uncovering a unique approach for damaged DNA replication: A computational investigation of a mutagenic tobacco-derived thymine lesion.Nucleic Acids Res. 2019 Feb 28;47(4):1871-1879. doi: 10.1093/nar/gky1265. Nucleic Acids Res. 2019. PMID: 30605521 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
