Overexpression of Srcin1 contributes to the growth and metastasis of colorectal cancer
- PMID: 28393242
- PMCID: PMC5403293
- DOI: 10.3892/ijo.2017.3952
Overexpression of Srcin1 contributes to the growth and metastasis of colorectal cancer
Abstract
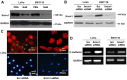
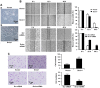
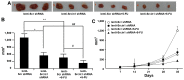
The adaptor protein Srcin1 is a novel Src-binding protein that regulates Src activation through C-terminal Src kinase (Csk). Srcin1 behaves as a tumour suppressor in breast cancer, but the role of Srcin1 in the development of colorectal cancer (CRC) remains unknown. In the present study, Srcin1 expression in normal tissue was examined by tissue microarray and assessed by immunohistochemistry in 10 patients. In addition, the biological impact of Srcin1 knockdown on CRC cells was investigated in vitro and in vivo. The results showed that Srcin1 was expressed in different types of normal human tissues, whereas its expression was increased in human CRC tissues. Srcin1 expression also correlated with tumour progression. The suppression of Srcin1 induced cell differentiation and G0/G1 cell cycle arrest. Furthermore, Srcin1 increased cell growth as well as the capacity of migration and invasion in CRC cells. Srcin1 induced the activation of the Wnt/β-catenin signalling pathway. Moreover, Srcin1 suppression sensitized cancer cells to 5-fluorouracil (5-FU)-induced apoptosis in vitro and in vivo. Together, these results demonstrate that Srcin1 contributes to CRC carcinogenesis, invasion and metastasis. These findings provide a rationale for a mechanistic approach to CRC treatment based on the development of Srcin1-targeted therapies.
Figures





References
-
- Chinery R, Beauchamp RD, Shyr Y, Kirkland SC, Coffey RJ, Morrow JD. Antioxidants reduce cyclooxygenase-2 expression, prostaglandin production, and proliferation in colorectal cancer cells. Cancer Res. 1998;58:2323–2327. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

