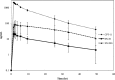
Determination of irinotecan, SN-38 and SN-38 glucuronide using HPLC/MS/MS: Application in a clinical pharmacokinetic and personalized medicine in colorectal cancer patients
- PMID: 28393405
- PMCID: PMC6817234
- DOI: 10.1002/jcla.22217
Determination of irinotecan, SN-38 and SN-38 glucuronide using HPLC/MS/MS: Application in a clinical pharmacokinetic and personalized medicine in colorectal cancer patients
Abstract
Background: Irinotecan (CPT-11) is chemotherapy used mainly in the metastatic colorectal cancer. The purpose of this study was to develop and validate the LC-MS/MS for the simultaneous determination of CPT-11, SN-38, and SN-38G.
Methods: A 100 μL of plasma was prepared after protein precipitation and analyzed on a C18 column using 0.1% acetic acid in water and 0.1% acetic acid in acetonitrile as mobile phases. The mass spectrometer worked with multiple reaction monitoring (MRM) in positive scan mode. The standard curves were linear on a concentration range of 5-10 000 ng/mL for CPT-11, 5-1000 ng/mL for SN-38, and 8-1000 ng/mL for SN-38G.
Results: In this assay, the intra and interday precision consisted of ≤9.11% and ≤11.29% for CPT-11, ≤8.70% and 8.31% for SN-38, and ≤9.90 and 9.64% for SN-38G.
Conclusion: This method was successfully used to quantify CPT-11, SN-38, and SN-38G and applied to a pharmacokinetic study.
Keywords: CPT-11; LC-MS/MS; SN-38; SN-38G; colorectal cancer.
© 2017 Wiley Periodicals, Inc.
Figures
Similar articles
-
Development and validation of a high-performance liquid chromatography-tandem mass spectrometry method for the simultaneous determination of irinotecan and its main metabolites in human plasma and its application in a clinical pharmacokinetic study.PLoS One. 2015 Feb 17;10(2):e0118194. doi: 10.1371/journal.pone.0118194. eCollection 2015. PLoS One. 2015. PMID: 25689738 Free PMC article.
-
Development and validation of an UPLC-MS/MS method for the quantification of irinotecan, SN-38 and SN-38 glucuronide in plasma, urine, feces, liver and kidney: Application to a pharmacokinetic study of irinotecan in rats.J Chromatogr B Analyt Technol Biomed Life Sci. 2016 Mar 15;1015-1016:34-41. doi: 10.1016/j.jchromb.2016.02.012. Epub 2016 Feb 9. J Chromatogr B Analyt Technol Biomed Life Sci. 2016. PMID: 26894853 Free PMC article.
-
Determination of irinotecan and its metabolite SN-38 in rabbit plasma and tumors using a validated method of tandem mass spectrometry coupled with liquid chromatography.J Chromatogr B Analyt Technol Biomed Life Sci. 2014 Jul 1;962:147-152. doi: 10.1016/j.jchromb.2014.05.042. Epub 2014 May 27. J Chromatogr B Analyt Technol Biomed Life Sci. 2014. PMID: 24927278
-
Irinotecan and its active metabolite, SN-38: review of bioanalytical methods and recent update from clinical pharmacology perspectives.Biomed Chromatogr. 2010 Jan;24(1):104-23. doi: 10.1002/bmc.1345. Biomed Chromatogr. 2010. PMID: 19852077 Review.
-
Clinical pharmacokinetics of irinotecan-based chemotherapy in colorectal cancer patients.Curr Clin Pharmacol. 2006 Sep;1(3):311-23. doi: 10.2174/157488406778249307. Curr Clin Pharmacol. 2006. PMID: 18666754 Review.
Cited by
-
Development and Application of Physiologically-Based Pharmacokinetic Model to Predict Systemic and Organ Exposure of Colorectal Cancer Drugs.Pharmaceutics. 2025 Jan 3;17(1):57. doi: 10.3390/pharmaceutics17010057. Pharmaceutics. 2025. PMID: 39861705 Free PMC article.
-
One-Step Solid Extraction for Simultaneous Determination of Eleven Commonly Used Anticancer Drugs and One Active Metabolite in Human Plasma by HPLC-MS/MS.J Anal Methods Chem. 2018 Jun 24;2018:7967694. doi: 10.1155/2018/7967694. eCollection 2018. J Anal Methods Chem. 2018. PMID: 30046507 Free PMC article.
-
Generation of anti-SN38 antibody for loading efficacy and therapeutic monitoring of SN38-containing therapeutics.Heliyon. 2024 Jun 18;10(12):e33232. doi: 10.1016/j.heliyon.2024.e33232. eCollection 2024 Jun 30. Heliyon. 2024. PMID: 39021912 Free PMC article.
-
Personalized drug stratification using endoscopic samples to assess ex vivo gastric cancer tissue susceptibility to chemotherapy and immune checkpoint inhibitors.Clin Exp Med. 2025 Jun 4;25(1):188. doi: 10.1007/s10238-025-01694-z. Clin Exp Med. 2025. PMID: 40465055 Free PMC article.
-
Rapid detection of the irinotecan-related UGT1A1*28 polymorphism by asymmetric PCR melting curve analysis using one fluorescent probe.J Clin Lab Anal. 2022 Aug;36(8):e24578. doi: 10.1002/jcla.24578. Epub 2022 Jun 29. J Clin Lab Anal. 2022. PMID: 35766440 Free PMC article.
References
-
- Newton KF, Newman W, Hill J. Review of biomarkers in colorectal cancer. Colorectal Dis. 2012;14:3‐17. - PubMed
-
- Di Paolo A, Bocci G, Polillo M, et al. Pharmacokinetic and pharmacogenetic predictive markers of irinotecan activity and toxicity. Curr Drug Metab. 2011;12:932‐943. - PubMed
-
- Rothenberg ML, Kuhn JG, Burris HA 3rd, et al. Phase I and pharmacokinetic trial of weekly CPT‐11. J Clin Oncol. 1993;11:2194‐2204. - PubMed
-
- Mathijssen RH, van Alphen RJ, Verweij J, et al. Clinical pharmacokinetics and metabolism of irinotecan (CPT‐11). Clin Cancer Res. 2001;7:2182‐2194. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical