Formal Synthesis of (+)-Laurencin by Gold(I)-Catalyzed Intramolecular Dehydrative Alkoxylation
- PMID: 28393406
- PMCID: PMC5653968
- DOI: 10.1002/chem.201700499
Formal Synthesis of (+)-Laurencin by Gold(I)-Catalyzed Intramolecular Dehydrative Alkoxylation
Abstract
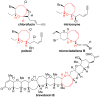
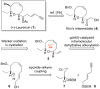
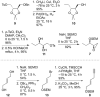
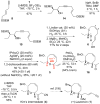
8-Membered cyclic ethers are found in a wide range of natural products; however, they are challenging synthetic targets due to enthalpic and entropic barriers. The gold(I)-catalyzed intramolecular dehydrative alkoxylation of ω-hydroxy allylic alcohols was explored to stereoselectively construct α,α'-cis-oxocenes and further applied in a formal synthesis of (+)-laurencin. The gold(I)-catalyzed intramolecular dehydrative alkoxylation may constitute an alternative method for the synthesis of molecular building blocks and natural products that contain highly functionalized 8-membered cyclic ethers.
Keywords: (+)-laurencin; dehydrative alkoxylation; gold catalysis; natural products; ω-hydroxy allylic alcohols.
© 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

Similar articles
-
[Reaction Development Utilizing the Features of Chemical Elements and Synthesis of Marine Natural Products].Yakugaku Zasshi. 2018;138(11):1335-1344. doi: 10.1248/yakushi.18-00126. Yakugaku Zasshi. 2018. PMID: 30381641 Review. Japanese.
-
The regio- and stereospecific intermolecular dehydrative alkoxylation of allylic alcohols catalyzed by a gold(I) N-heterocyclic carbene complex.Chemistry. 2013 Mar 4;19(10):3437-44. doi: 10.1002/chem.201203987. Epub 2013 Jan 24. Chemistry. 2013. PMID: 23348826 Free PMC article.
-
Asymmetric total syntheses of (+)-3-(z)-laureatin and (+)-3-(z)-isolaureatin by "lone pair-lone pair interaction-controlled" isomerization.J Am Chem Soc. 2007 Feb 28;129(8):2269-74. doi: 10.1021/ja068346i. Epub 2007 Feb 6. J Am Chem Soc. 2007. PMID: 17279755
-
Highly stereoselective and efficient total synthesis of (+)-laurencin.Org Lett. 2005 Jan 6;7(1):75-7. doi: 10.1021/ol047877d. Org Lett. 2005. PMID: 15624981
-
[Convergent Synthesis of Fused Ring Systems in Large Polycyclic Ethers].Yakugaku Zasshi. 2017;137(9):1095-1101. doi: 10.1248/yakushi.17-00116. Yakugaku Zasshi. 2017. PMID: 28867696 Review. Japanese.
Cited by
-
Naturally Occurring Organohalogen Compounds-A Comprehensive Review.Prog Chem Org Nat Prod. 2023;121:1-546. doi: 10.1007/978-3-031-26629-4_1. Prog Chem Org Nat Prod. 2023. PMID: 37488466 Review.
References
-
- Widenhoefer RA, Han XQ. Eur J Org Chem. 2006:4555–4563.
-
- Muñoz MP. Chem Soc Rev. 2014;43:3164–3183. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

