Formal Synthesis of (+)-Laurencin by Gold(I)-Catalyzed Intramolecular Dehydrative Alkoxylation
- PMID: 28393406
- PMCID: PMC5653968
- DOI: 10.1002/chem.201700499
Formal Synthesis of (+)-Laurencin by Gold(I)-Catalyzed Intramolecular Dehydrative Alkoxylation
Abstract
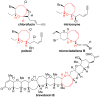
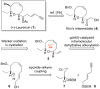
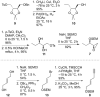
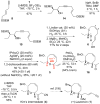
8-Membered cyclic ethers are found in a wide range of natural products; however, they are challenging synthetic targets due to enthalpic and entropic barriers. The gold(I)-catalyzed intramolecular dehydrative alkoxylation of ω-hydroxy allylic alcohols was explored to stereoselectively construct α,α'-cis-oxocenes and further applied in a formal synthesis of (+)-laurencin. The gold(I)-catalyzed intramolecular dehydrative alkoxylation may constitute an alternative method for the synthesis of molecular building blocks and natural products that contain highly functionalized 8-membered cyclic ethers.
Keywords: (+)-laurencin; dehydrative alkoxylation; gold catalysis; natural products; ω-hydroxy allylic alcohols.
© 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

References
-
- Widenhoefer RA, Han XQ. Eur J Org Chem. 2006:4555–4563.
-
- Muñoz MP. Chem Soc Rev. 2014;43:3164–3183. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

