Involvement of a velvet protein ClVelB in the regulation of vegetative differentiation, oxidative stress response, secondary metabolism, and virulence in Curvularia lunata
- PMID: 28393907
- PMCID: PMC5385503
- DOI: 10.1038/srep46054
Involvement of a velvet protein ClVelB in the regulation of vegetative differentiation, oxidative stress response, secondary metabolism, and virulence in Curvularia lunata
Abstract
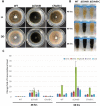
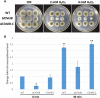
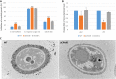
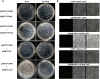
The ortholog of Aspergillus nidulans VelB, which is known as ClVelB, was studied to gain a broader insight into the functions of a velvet protein in Curvularia lunata. With the expected common and specific functions of ClVelB, the deletion of clvelB results in similar though not identical phenotypes. The pathogenicity assays revealed that ΔClVelB was impaired in colonizing the host tissue, which corresponds to the finding that ClVelB controls the production of conidia and the methyl 5-(hydroxymethyl) furan-2-carboxylate toxin in C. lunata. However, the deletion of clvelB led to the increase in aerial hyphae and melanin formation. In addition, ΔClVelB showed a decreased sensitivity to iprodione and fludioxonil fungicides and a decreased resistance to cell wall-damaging agents and osmotic stress and tolerance to H2O2. The ultrastructural analysis indicated that the cell wall of ΔClVelB became thinner, which agrees with the finding that the accumulated level of glycerol in ΔClVelB is lower than the wild-type. Furthermore, the interaction of ClVelB with ClVeA and ClVosA was identified in the present research through the yeast two-hybrid and bimolecular fluorescence complementation assays. Results indicate that ClVelB plays a vital role in the regulation of various cellular processes in C. lunata.
Conflict of interest statement
The authors declare no competing financial interests.
Figures









Similar articles
-
Involvement of a velvet protein FgVeA in the regulation of asexual development, lipid and secondary metabolisms and virulence in Fusarium graminearum.PLoS One. 2011;6(11):e28291. doi: 10.1371/journal.pone.0028291. Epub 2011 Nov 29. PLoS One. 2011. PMID: 22140571 Free PMC article.
-
StPBS2, a MAPK kinase gene, is involved in determining hyphal morphology, cell wall development, hypertonic stress reaction as well as the production of secondary metabolites in Northern Corn Leaf Blight pathogen Setosphaeria turcica.Microbiol Res. 2017 Aug;201:30-38. doi: 10.1016/j.micres.2017.04.009. Epub 2017 Apr 26. Microbiol Res. 2017. PMID: 28602399
-
The VELVET Complex in the Gray Mold Fungus Botrytis cinerea: Impact of BcLAE1 on Differentiation, Secondary Metabolism, and Virulence.Mol Plant Microbe Interact. 2015 Jun;28(6):659-74. doi: 10.1094/MPMI-12-14-0411-R. Epub 2015 Jun 23. Mol Plant Microbe Interact. 2015. PMID: 25625818
-
Global insight into the distribution of velvet-like B protein in Cochliobolus species and implication in pathogenicity and fungicide resistance.World J Microbiol Biotechnol. 2018 Dec 1;34(12):187. doi: 10.1007/s11274-018-2569-6. World J Microbiol Biotechnol. 2018. PMID: 30506400 Review.
-
[Progress in the study of Velvet and LaeA proteins and their relation to the development and bioactive compounds in medicinal fungi].Yao Xue Xue Bao. 2014 Nov;49(11):1520-7. Yao Xue Xue Bao. 2014. PMID: 25757276 Review. Chinese.
Cited by
-
The velvet family proteins mediate low resistance to isoprothiolane in Magnaporthe oryzae.PLoS Pathog. 2023 Jun 5;19(6):e1011011. doi: 10.1371/journal.ppat.1011011. eCollection 2023 Jun. PLoS Pathog. 2023. PMID: 37276223 Free PMC article.
-
Beyond morphogenesis and secondary metabolism: function of Velvet proteins and LaeA in fungal pathogenesis.Appl Environ Microbiol. 2024 Oct 23;90(10):e0081924. doi: 10.1128/aem.00819-24. Epub 2024 Sep 4. Appl Environ Microbiol. 2024. PMID: 39230285 Free PMC article. Review.
-
Involvement of LaeA and Velvet Proteins in Regulating the Production of Mycotoxins and Other Fungal Secondary Metabolites.J Fungi (Basel). 2024 Aug 8;10(8):561. doi: 10.3390/jof10080561. J Fungi (Basel). 2024. PMID: 39194887 Free PMC article. Review.
-
The ZtvelB Gene Is Required for Vegetative Growth and Sporulation in the Wheat Pathogen Zymoseptoria tritici.Front Microbiol. 2019 Oct 1;10:2210. doi: 10.3389/fmicb.2019.02210. eCollection 2019. Front Microbiol. 2019. PMID: 31632366 Free PMC article.
-
Gene expression, molecular docking, and molecular dynamics studies to identify potential antifungal compounds targeting virulence proteins/genes VelB and THR as possible drug targets against Curvularia lunata.Front Mol Biosci. 2022 Dec 12;9:1055945. doi: 10.3389/fmolb.2022.1055945. eCollection 2022. Front Mol Biosci. 2022. PMID: 36619165 Free PMC article.
References
-
- Liu T., Liu L. X., Jiang X., Huang X. L. & Chen J. A new furanoid toxin produced by Curvularia lunata, the causal agent of maize Curvularia leaf spot. Can. J. Plant Pathol. 31, 22–27 (2009).
-
- Gao J. X., Liu T. & Chen J. Insertional mutagenesis and cloning of the gene required for the biosynthesis of the non-host specific toxin in Cochliobolus lunatus that causes maize leaf spot. Phytopathology 104(4), 332–339 (2014). - PubMed
-
- Gao S. G. et al. Understanding resistant germplasm-induced virulence variation through analysis of proteomics and suppression subtractive hybridization in a maize pathogen Curvularia lunata. Proteomics 12, 1–12 (2012). - PubMed
-
- Merhej J., Richard-Forget F. & Barreau C. Regulation of trichothecene biosynthesis in Fusarium: recent advances and new insights. Appl. Microbiol. Biotechnol. 91, 519–528 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

