Light-assisted delithiation of lithium iron phosphate nanocrystals towards photo-rechargeable lithium ion batteries
- PMID: 28393912
- PMCID: PMC5394232
- DOI: 10.1038/ncomms14643
Light-assisted delithiation of lithium iron phosphate nanocrystals towards photo-rechargeable lithium ion batteries
Abstract
Recently, intensive efforts are dedicated to convert and store the solar energy in a single device. Herein, dye-synthesized solar cell technology is combined with lithium-ion materials to investigate light-assisted battery charging. In particular we report the direct photo-oxidation of lithium iron phosphate nanocrystals in the presence of a dye as a hybrid photo-cathode in a two-electrode system, with lithium metal as anode and lithium hexafluorophosphate in carbonate-based electrolyte; a configuration corresponding to lithium ion battery charging. Dye-sensitization generates electron-hole pairs with the holes aiding the delithiation of lithium iron phosphate at the cathode and electrons utilized in the formation of a solid electrolyte interface at the anode via oxygen reduction. Lithium iron phosphate acts effectively as a reversible redox agent for the regeneration of the dye. Our findings provide possibilities in advancing the design principles for photo-rechargeable lithium ion batteries.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

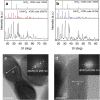
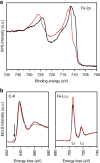

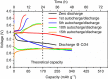


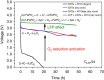
References
-
- Yu M. et al.. Solar-powered electrochemical energy storage: an alternative to solar fuels. J. Mater. Chem. A 4, 2766–2782 (2016).
-
- Schmidt D. et al.. Photo-rechargeable electric energy storage systems. Adv. Energy Mater. 6, 1–11 (2016).
-
- Hodes G. et al.. Photoelectrochemical energy conversion and storage using polycrystalline chalcogenide electrodes. Nature 261, 403–404 (1976).
-
- Manassen J. et al.. Photoelectrochemical energy conversion and storage. J. Electrochem. Soc. 124, 532–534 (1977).
-
- Kanbara T. et al.. Photo-rechargeable solid state battery. Solid State Ionics 40, 955–958 (1990).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

