The neural correlates of dreaming
- PMID: 28394322
- PMCID: PMC5462120
- DOI: 10.1038/nn.4545
The neural correlates of dreaming
Abstract
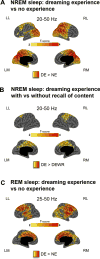
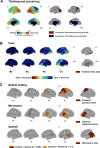
Consciousness never fades during waking. However, when awakened from sleep, we sometimes recall dreams and sometimes recall no experiences. Traditionally, dreaming has been identified with rapid eye-movement (REM) sleep, characterized by wake-like, globally 'activated', high-frequency electroencephalographic activity. However, dreaming also occurs in non-REM (NREM) sleep, characterized by prominent low-frequency activity. This challenges our understanding of the neural correlates of conscious experiences in sleep. Using high-density electroencephalography, we contrasted the presence and absence of dreaming in NREM and REM sleep. In both NREM and REM sleep, reports of dream experience were associated with local decreases in low-frequency activity in posterior cortical regions. High-frequency activity in these regions correlated with specific dream contents. Monitoring this posterior 'hot zone' in real time predicted whether an individual reported dreaming or the absence of dream experiences during NREM sleep, suggesting that it may constitute a core correlate of conscious experiences in sleep.
Conflict of interest statement
The other authors have indicated no financial conflicts of interest.
Figures



Comment in
-
Unraveling the mystery of dreams.J Thorac Dis. 2017 Sep;9(9):2732-2735. doi: 10.21037/jtd.2017.07.103. J Thorac Dis. 2017. PMID: 29221226 Free PMC article. No abstract available.
References
-
- Stickgold R, Malia A, Fosse R, Propper R, Hobson JA. Brain-mind states: I. Longitudinal field study of sleep/wake factors influencing mentation report length. Sleep. 2001;24:171–179. - PubMed
-
- Schwartz S, Maquet P. Sleep imaging and the neuro-psychological assessment of dreams. Trends Cogn Sci. 2002;6:23–30. - PubMed
-
- Aserinsky E, Kleitman N. Regularly occuring periods of eye motility, and concominant phenomena, during sleep. Science. 1953;118:273–274. - PubMed
-
- Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1:455–473. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

