Sharp wave ripples during learning stabilize the hippocampal spatial map
- PMID: 28394323
- PMCID: PMC5446786
- DOI: 10.1038/nn.4543
Sharp wave ripples during learning stabilize the hippocampal spatial map
Abstract
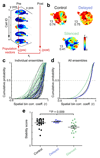
Cognitive representation of the environment requires a stable hippocampal map, but the mechanisms maintaining a given map are unknown. Because sharp wave-ripples (SPW-R) orchestrate both retrospective and prospective spatial information, we hypothesized that disrupting neuronal activity during SPW-Rs affects spatial representation. Mice learned new sets of three goal locations daily in a multiwell maze. We used closed-loop SPW-R detection at goal locations to trigger optogenetic silencing of a subset of CA1 pyramidal neurons. Control place cells (nonsilenced or silenced outside SPW-Rs) largely maintained the location of their place fields after learning and showed increased spatial information content. In contrast, the place fields of SPW-R-silenced place cells remapped, and their spatial information remained unaltered. SPW-R silencing did not impact the firing rates or proportions of place cells. These results suggest that interference with SPW-R-associated activity during learning prevents stabilization and refinement of hippocampal maps.
Figures





References
-
- O'Keefe J, Nadel L. Book The Hippocampus as a Cognitive Map. Oxford University Press; 1978.
-
- Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–1058. - PubMed
-
- Buzsaki G, Leung LW, Vanderwolf CH. Cellular bases of hippocampal EEG in the behaving rat. Brain Res. 1983;287:139–171. - PubMed
-
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

