Analysis of host microRNA function uncovers a role for miR-29b-2-5p in Shigella capture by filopodia
- PMID: 28394930
- PMCID: PMC5398735
- DOI: 10.1371/journal.ppat.1006327
Analysis of host microRNA function uncovers a role for miR-29b-2-5p in Shigella capture by filopodia
Abstract
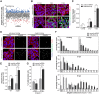
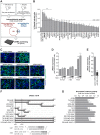
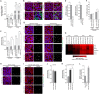
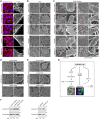
MicroRNAs play an important role in the interplay between bacterial pathogens and host cells, participating as host defense mechanisms, as well as exploited by bacteria to subvert host cellular functions. Here, we show that microRNAs modulate infection by Shigella flexneri, a major causative agent of bacillary dysentery in humans. Specifically, we characterize the dual regulatory role of miR-29b-2-5p during infection, showing that this microRNA strongly favors Shigella infection by promoting both bacterial binding to host cells and intracellular replication. Using a combination of transcriptome analysis and targeted high-content RNAi screening, we identify UNC5C as a direct target of miR-29b-2-5p and show its pivotal role in the modulation of Shigella binding to host cells. MiR-29b-2-5p, through repression of UNC5C, strongly enhances filopodia formation thus increasing Shigella capture and promoting bacterial invasion. The increase of filopodia formation mediated by miR-29b-2-5p is dependent on RhoF and Cdc42 Rho-GTPases. Interestingly, the levels of miR-29b-2-5p, but not of other mature microRNAs from the same precursor, are decreased upon Shigella replication at late times post-infection, through degradation of the mature microRNA by the exonuclease PNPT1. While the relatively high basal levels of miR-29b-2-5p at the start of infection ensure efficient Shigella capture by host cell filopodia, dampening of miR-29b-2-5p levels later during infection may constitute a bacterial strategy to favor a balanced intracellular replication to avoid premature cell death and favor dissemination to neighboring cells, or alternatively, part of the host response to counteract Shigella infection. Overall, these findings reveal a previously unappreciated role of microRNAs, and in particular miR-29b-2-5p, in the interaction of Shigella with host cells.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
The Human-Specific miR-6762-5p Is an Activator of RhoA GTPase Enhancing Shigella flexneri Intercellular Spreading.Mol Microbiol. 2025 May;123(5):420-432. doi: 10.1111/mmi.15352. Epub 2025 Feb 24. Mol Microbiol. 2025. PMID: 39992886 Free PMC article.
-
Lentivirus-mediated Bos taurus bta-miR-29b overexpression interferes with bovine viral diarrhoea virus replication and viral infection-related autophagy by directly targeting ATG14 and ATG9A in Madin-Darby bovine kidney cells.J Gen Virol. 2015 Jan;96(Pt 1):85-94. doi: 10.1099/vir.0.067140-0. Epub 2014 Sep 18. J Gen Virol. 2015. PMID: 25234643
-
Genome-wide mRNA-miRNA profiling uncovers a role of the microRNA miR-29b-1-5p/PHLPP1 signalling pathway in Helicobacter pylori-driven matrix metalloproteinase production in gastric epithelial cells.Cell Microbiol. 2018 Sep;20(9):e12859. doi: 10.1111/cmi.12859. Epub 2018 May 25. Cell Microbiol. 2018. PMID: 29749704
-
[Pathogenesis of Shigella: the study of bacteria-host interplay at the intestinal mucosal barriers].Nihon Saikingaku Zasshi. 2012;67(4):257-68. doi: 10.3412/jsb.67.257. Nihon Saikingaku Zasshi. 2012. PMID: 23269180 Review. Japanese.
-
A new paradigm of bacteria-gut interplay brought through the study of Shigella.Proc Jpn Acad Ser B Phys Biol Sci. 2010;86(3):229-43. doi: 10.2183/pjab.86.229. Proc Jpn Acad Ser B Phys Biol Sci. 2010. PMID: 20228623 Free PMC article. Review.
Cited by
-
Functional screenings reveal different requirements for host microRNAs in Salmonella and Shigella infection.Nat Microbiol. 2020 Jan;5(1):192-205. doi: 10.1038/s41564-019-0614-3. Epub 2019 Dec 2. Nat Microbiol. 2020. PMID: 31792428
-
miR-215 Modulates Ubiquitination to Impair Inflammasome Activation and Autophagy During Salmonella Typhimurium Infection in Porcine Intestinal Cells.Animals (Basel). 2025 Feb 4;15(3):431. doi: 10.3390/ani15030431. Animals (Basel). 2025. PMID: 39943201 Free PMC article.
-
Long Noncoding RNA LOC550643 Acts as an Oncogene in the Growth Regulation of Colorectal Cancer Cells.Cells. 2022 Mar 22;11(7):1065. doi: 10.3390/cells11071065. Cells. 2022. PMID: 35406629 Free PMC article.
-
Multifaceted Roles of microRNAs in Host-Bacterial Pathogen Interaction.Microbiol Spectr. 2019 May;7(3):10.1128/microbiolspec.bai-0002-2019. doi: 10.1128/microbiolspec.BAI-0002-2019. Microbiol Spectr. 2019. PMID: 31152522 Free PMC article. Review.
-
Understanding microRNAs in the Context of Infection to Find New Treatments against Human Bacterial Pathogens.Antibiotics (Basel). 2022 Mar 8;11(3):356. doi: 10.3390/antibiotics11030356. Antibiotics (Basel). 2022. PMID: 35326819 Free PMC article. Review.
References
-
- Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382(9888):209–22. 10.1016/S0140-6736(13)60844-2 - DOI - PubMed
-
- Brotcke Zumsteg A, Goosmann C, Brinkmann V, Morona R, Zychlinsky A. IcsA is a Shigella flexneri adhesin regulated by the type III secretion system and required for pathogenesis. Cell host & microbe. 2014;15(4):435–45. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

