Identification of a rice metal tolerance protein OsMTP11 as a manganese transporter
- PMID: 28394944
- PMCID: PMC5386239
- DOI: 10.1371/journal.pone.0174987
Identification of a rice metal tolerance protein OsMTP11 as a manganese transporter
Abstract
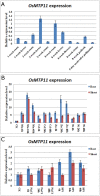
Metal tolerance proteins (MTPs) are a gene family of cation efflux transporters that occur widely in plants and might serve an essential role in metal homeostasis and tolerance. Our research describes the identification, characterization, and localization of OsMTP11, a member of the MTP family from rice. OsMTP11 was expressed constitutively and universally in different tissues in rice plant. Heterologous expression in yeast showed that OsMTP11 complemented the hypersensitivity of mutant strains to Mn, and also complemented yeast mutants to other metals, including Co and Ni. Real time RT-PCR analysis demonstrated OsMTP11 expression was substantially enhanced following 4 h under Cd, Zn, Ni, and Mn treatments, suggesting possible roles of OsMTP11 involvement in heavy metal stress responses. Promoter analysis by transgenic assays with GUS as a reporter gene and mRNA in situ hybridization experiments showed that OsMTP11 was expressed specifically in conducting tissues in rice. DNA methylation assays of genomic DNA in rice treated with Cd, Zn, Ni, and Mn revealed that decreased DNA methylation levels were present in the OsMTP11 promoter region, which was consistent with OsMTP11 induced-expression patterns resulting from heavy metal stress. This result suggested that DNA methylation is one of major factors regulating expression of OsMTP11 through epigenetic mechanisms. OsMTP11 fused to green fluorescent protein (GFP) localized to the entire onion epidermal cell cytoplasm, while vacuolar membrane exhibited increased GFP signals, consistent with an OsMTP11 function in cation sequestration. Our results indicated that OsMTP11 might play vital roles in Mn and other heavy metal transportation in rice.
Conflict of interest statement
Figures








Similar articles
-
OsMTP11, a trans-Golgi network localized transporter, is involved in manganese tolerance in rice.Plant Sci. 2018 Sep;274:59-69. doi: 10.1016/j.plantsci.2018.05.011. Epub 2018 May 17. Plant Sci. 2018. PMID: 30080641
-
OsMTP11 is localised at the Golgi and contributes to Mn tolerance.Sci Rep. 2017 Nov 10;7(1):15258. doi: 10.1038/s41598-017-15324-6. Sci Rep. 2017. PMID: 29127328 Free PMC article.
-
Molecular characterization of a rice metal tolerance protein, OsMTP1.Plant Cell Rep. 2012 Jan;31(1):67-79. doi: 10.1007/s00299-011-1140-9. Epub 2011 Sep 3. Plant Cell Rep. 2012. PMID: 21892614
-
[Roles of Zinc Transporters That Control the Essentiality and Toxicity of Manganese and Cadmium].Yakugaku Zasshi. 2021;141(5):695-703. doi: 10.1248/yakushi.20-00243-5. Yakugaku Zasshi. 2021. PMID: 33952754 Review. Japanese.
-
Managing the manganese: molecular mechanisms of manganese transport and homeostasis.New Phytol. 2005 Sep;167(3):733-42. doi: 10.1111/j.1469-8137.2005.01453.x. New Phytol. 2005. PMID: 16101910 Review.
Cited by
-
A Golgi-Located Transmembrane Nine Protein Gene TMN11 Functions in Manganese/Cadmium Homeostasis and Regulates Growth and Seed Development in Rice.Int J Mol Sci. 2022 Dec 14;23(24):15883. doi: 10.3390/ijms232415883. Int J Mol Sci. 2022. PMID: 36555524 Free PMC article.
-
Manganese in Plants: From Acquisition to Subcellular Allocation.Front Plant Sci. 2020 Mar 26;11:300. doi: 10.3389/fpls.2020.00300. eCollection 2020. Front Plant Sci. 2020. PMID: 32273877 Free PMC article. Review.
-
Complex Gene Regulation Underlying Mineral Nutrient Homeostasis in Soybean Root Response to Acidity Stress.Genes (Basel). 2019 May 27;10(5):402. doi: 10.3390/genes10050402. Genes (Basel). 2019. PMID: 31137896 Free PMC article.
-
Genome-wide association mapping for grain manganese in rice (Oryza sativa L.) using a multi-experiment approach.Heredity (Edinb). 2021 Mar;126(3):505-520. doi: 10.1038/s41437-020-00390-w. Epub 2020 Nov 24. Heredity (Edinb). 2021. PMID: 33235293 Free PMC article.
-
Comparative Transcriptome Analysis Reveals Complex Physiological Response and Gene Regulation in Peanut Roots and Leaves under Manganese Toxicity Stress.Int J Mol Sci. 2023 Jan 6;24(2):1161. doi: 10.3390/ijms24021161. Int J Mol Sci. 2023. PMID: 36674676 Free PMC article.
References
-
- Mäser P, Thomine S, Schroeder JI, Ward JM, Hirschi K, Sze H, Talke IN, Amtmann A, Maathuis FJ, Sanders D, Harper JF, Tchieu J, Gribskov M, Persans MW, Salt DE, Kim SA, Guerinot ML. Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol. 2001; 126: 1646–1667. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

