Inhibition of Stat3 by a Small Molecule Inhibitor Slows Vision Loss in a Rat Model of Diabetic Retinopathy
- PMID: 28395025
- PMCID: PMC5386345
- DOI: 10.1167/iovs.16-20641
Inhibition of Stat3 by a Small Molecule Inhibitor Slows Vision Loss in a Rat Model of Diabetic Retinopathy
Abstract
Purpose: Diabetic retinopathy is a leading cause of vision loss. Previous studies have shown signaling pathways mediated by Stat3 (signal transducer and activator of transcription 3) play a primary role in diabetic retinopathy progression. This study tested CLT-005, a small molecule inhibitor of Stat3, for its dose-dependent therapeutic effects on vision loss in a rat model of diabetic retinopathy.
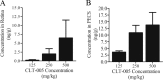
Methods: Brown Norway rats were administered streptozotocin (STZ) to induce diabetes. CLT-005 was administered daily by oral gavage for 16 weeks at concentrations of 125, 250, or 500 mg/kg, respectively, beginning 4 days post streptozotocin administration. Systemic and ocular drug concentration was quantified with mass spectrometry. Visual function was monitored at 2-week intervals from 6 to 16 weeks using optokinetic tracking to measure visual acuity and contrast sensitivity. The presence and severity of cataracts was visually monitored and correlated to visual acuity. The transcription and translation of multiple angiogenic factors and inflammatory cytokines were measured by real-time polymerase chain reaction and Multiplex immunoassay.
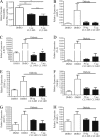
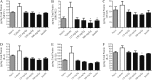
Results: Streptozotocin-diabetic rats sustain progressive vision loss over 16 weeks, and this loss in visual function is rescued in a dose-dependent manner by CLT-005. This positive therapeutic effect correlates to the positive effects of CLT-005 on vascular leakage and the presence of inflammatory cytokines in the retina.
Conclusions: The present study indicates that Stat3 inhibition has strong therapeutic potential for the treatment of vision loss in diabetic retinopathy.
Figures



Similar articles
-
Strain-dependent increases in retinal inflammatory proteins and photoreceptor FGF-2 expression in streptozotocin-induced diabetic rats.Invest Ophthalmol Vis Sci. 2009 Nov;50(11):5396-404. doi: 10.1167/iovs.09-3474. Epub 2009 May 27. Invest Ophthalmol Vis Sci. 2009. PMID: 19474406
-
Progressive Early Breakdown of Retinal Pigment Epithelium Function in Hyperglycemic Rats.Invest Ophthalmol Vis Sci. 2016 May 1;57(6):2706-13. doi: 10.1167/iovs.15-18397. Invest Ophthalmol Vis Sci. 2016. PMID: 27191823 Free PMC article.
-
TrkB signalling pathway mediates the protective effects of exercise in the diabetic rat retina.Eur J Neurosci. 2018 May;47(10):1254-1265. doi: 10.1111/ejn.13909. Epub 2018 Apr 3. Eur J Neurosci. 2018. PMID: 29537701 Free PMC article.
-
Preventing blindness due to diabetic retinopathy. Control glycaemia and blood pressure, and monitor the eyes.Prescrire Int. 2010 Feb;19(105):35-8. Prescrire Int. 2010. PMID: 20455344 Review.
-
Fibrates and statins in the treatment of diabetic retinopathy.Curr Pharm Biotechnol. 2011 Mar 1;12(3):396-405. doi: 10.2174/138920111794480570. Curr Pharm Biotechnol. 2011. PMID: 20939802 Review.
Cited by
-
Hu-Zhang Qing-Mai Formulation anti-oxidative stress alleviates diabetic retinopathy: Network pharmacology analysis and in vitro experiment.Medicine (Baltimore). 2023 Sep 8;102(36):e35034. doi: 10.1097/MD.0000000000035034. Medicine (Baltimore). 2023. PMID: 37682156 Free PMC article.
-
CircZNF532 knockdown protects retinal pigment epithelial cells against high glucose-induced apoptosis and pyroptosis by regulating the miR-20b-5p/STAT3 axis.J Diabetes Investig. 2022 May;13(5):781-795. doi: 10.1111/jdi.13722. Epub 2022 Feb 11. J Diabetes Investig. 2022. PMID: 34839589 Free PMC article.
-
Modulation of Oxidative Stress in Diabetic Retinopathy: Therapeutic Role of Natural Polyphenols.Antioxidants (Basel). 2025 Jul 17;14(7):875. doi: 10.3390/antiox14070875. Antioxidants (Basel). 2025. PMID: 40722979 Free PMC article. Review.
-
A Narrative Review of STAT Proteins in Diabetic Retinopathy: From Mechanisms to Therapeutic Prospects.Ophthalmol Ther. 2022 Dec;11(6):2005-2026. doi: 10.1007/s40123-022-00581-0. Epub 2022 Oct 8. Ophthalmol Ther. 2022. PMID: 36208390 Free PMC article. Review.
-
Microglial Activation Is Associated With Vasoprotection in a Rat Model of Inflammatory Retinal Vasoregression.Front Physiol. 2021 Apr 26;12:660164. doi: 10.3389/fphys.2021.660164. eCollection 2021. Front Physiol. 2021. PMID: 33981252 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

