Contributions to the dynamics of cervix remodeling prior to term and preterm birth
- PMID: 28395330
- PMCID: PMC5803764
- DOI: 10.1095/biolreprod.116.142844
Contributions to the dynamics of cervix remodeling prior to term and preterm birth
Abstract
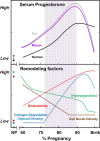
Major clinical challenges for obstetricians and neonatologists result from early cervix remodeling and preterm birth. Complications related to cervix remodeling or delivery account for significant morbidity in newborns and peripartum mothers. Understanding morphology and structure of the cervix in pregnant women is limited mostly to the period soon before and after birth. However, evidence in rodent models supports a working hypothesis that a convergence of factors promotes a physiological inflammatory process that degrades the extracellular collagen matrix and enhances biomechanical distensibility of the cervix well before the uterus develops the contractile capabilities for labor. Contributing factors to this remodeling process include innervation, mechanical stretch, hypoxia, and proinflammatory mediators. Importantly, the softening and shift to ripening occurs while progesterone is near peak concentrations in circulation across species. Since progesterone is required to maintain pregnancy, the premise of this review is that loss of responsiveness to progesterone constitutes a common final mechanism for remodeling the mammalian cervix in preparation for birth at term. Various inputs are suggested to promote signaling between stromal cells and resident macrophages to drive proinflammatory processes that advance the soft cervix into ripening. With infection, pathophysiological processes may prematurely drive components of this remodeling mechanism and lead to preterm birth. Identification of critical molecules and pathways from studies in various rodent models hold promise for novel endpoints to assess risk and provide innovative approaches to treat preterm birth or promote the progress of ripening at term.
Keywords: cervix; extracellular matrix; macrophage; parturition; progesterone/progesterone receptor.
© The Author 2016. Published by Oxford University Press on behalf of Society for the Study of Reproduction.
Figures

References
-
- Martin JA, Hamilton BE, Osterman MJ. Births in the United States, 2014. NCHS Data Brief 2015; 216:1–8. - PubMed
-
- Iams JD, Goldenberg RL, Meis PJ, Mercer BM, Moawad A, Das A, Thom E, McNellis D, Copper RL, Johnson F, Roberts JM. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med 1996; 334:567–572. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

