[Prognostic significance of blood count at the time of achieving morphologic leukemia-free state in adults with acute myeloid leukemia]
- PMID: 28395440
- PMCID: PMC7348386
- DOI: 10.3760/cma.j.issn.0253-2727.2017.03.003
[Prognostic significance of blood count at the time of achieving morphologic leukemia-free state in adults with acute myeloid leukemia]
Abstract
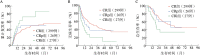
Objective: To explore prognostic significance of blood count at the time of achieving first morphologic leukemia-free state[complete remission (CR, ANC ≥1×10(9)/L and PLT ≥100×10(9)/L) , CR with incomplete PLT recovery (CRp) and CR with incomplete ANC and PLT recovery (CRi) ]in adult patients with de novo acute myeloid leukemia (AML) . Methods: From January 2008 to February 2016, data of consecutive newly-diagnosed AML (non-APL) adults who received continuous chemotherapy in our hospital were analyzed retrospectively. Results: 352 patients were included in the study. 179 (50.9%) were male. Median age was 44 (17-65) years. Using the SWOG cytogenetic classification, 87 (24.7%) , 171 (48.6%) , 46 (13.1%) and 48 (13.6%) patients belonged to favorable, intermediate, unfavorable and unknown categories, respectively. 16 (4.5%) had monosomal karyotype and 41 (11.6%) had FLT3-ITD mutation positive. Best response achieved at the time of achieving first morphologic leukemia-free state was CR in 299 (84.9%) patients, CRp in 26 (7.4%) and CRi in 27 (8.1%) . With a median follow-up period of 16 (2-94) months in survivors, the probabilities of cumulative incident of relapse (CIR) rate, disease free survival (DFS) and overall survival (OS) at 30 months were 47.5%, 46.0% and 58.6%, respectively. Multivariate analyses showed that non-CR (CRp or CRi) , was associated with high relapse rate, shorter DFS and OS. In addition, intermediate or high risk of SWOG cytogenetic classification and FLT3-ITD positive were common unfavorable factors affecting CIR, DFS and OS. Peripheral blast ≥60% at diagnosis was adverse factors affecting DFS. Age ≥48 years and bone marrow blasts ≥67% were associated with shorter OS. Conclusion: Blood count at the time of achieving morphologic leukemia-free state was one of the key markers associated with treatment outcomes in adults with AML.
目的:探讨首次获骨髓无白血病状态时血细胞恢复程度[包括完全缓解(CR,ANC≥1.0× 10(9)/L和PLT≥100×10(9)/L)、PLT未恢复(CRp)、ANC和PLT均未恢复(CRi)]对初治成人急性髓系白血病(AML)患者预后的影响。方法:回顾2008年1月至2016年2月北京大学人民医院收治的获得骨髓无白血病状态后持续化疗AML(非急性早幼粒细胞白血病)连续病例,分析诊断时疾病特征、诱导化疗方案、首次诱导化疗反应以及骨髓无白血病状态时血细胞计数与预后的关系。结果: 352例患者,男179例(50.9%),中位年龄44(17~65)岁。按美国西南肿瘤组(SWOG)标准分组:低危87例(24.7%),中危171例(48.6%),高危46例(13.1%),未知48例(13.6%)。单体核型16例(4.5%),FLT3-ITD突变阳性41例(11.6%)。首次获骨髓无白血病状态时血细胞恢复程度:CR 299例(84.9%),CRp 26例(7.4%),CRi 27例(7.7%)。存活患者中位随访16(2~94)个月,30个月累积复发(CIR)、无病生存(DFS)和总生存(OS)率分别为47.5%、46.0%和58.6%。多因素分析显示,骨髓无白血病状态时血细胞恢复不良是影响患者CIR、DFS和OS的共同不利因素(HR=1.4,95% CI 1.0~1.9,P=0.037;HR=1.5,95% CI 1.1~2.0,P= 0.003;HR=1.5,95% CI 1.1~2.0,P=0.017)。此外,SWOG分组危险度高和FLT3-ITD突变阳性是影响患者CIR、DFS和OS的共同不利因素;确诊时外周血原始细胞比例高是影响患者DFS的不利因素;年龄大和确诊时骨髓原始细胞比例高是影响患者OS的不利因素。结论:持续化疗的成人AML患者,首次获骨髓无白血病状态时血细胞恢复程度是影响预后的独立因素。.
Keywords: Blood cell count; Leukemia, myeloid, acute; Prognosis; Remission induction.
Figures
Similar articles
-
[Outcomes of adult patients with de novo acute myeloid leukemia received idarubicin plus cytarabine regimen as induction chemotherapy].Zhonghua Xue Ye Xue Za Zhi. 2018 Jan 14;39(1):15-21. doi: 10.3760/cma.j.issn.0253-2727.2018.01.004. Zhonghua Xue Ye Xue Za Zhi. 2018. PMID: 29551027 Free PMC article. Chinese.
-
[Minimal residual disease level predicts outcomes in the non-favorable risk patients with acute myeloid leukemia].Zhonghua Xue Ye Xue Za Zhi. 2017 Jul 14;38(7):578-585. doi: 10.3760/cma.j.issn.0253-2727.2017.07.005. Zhonghua Xue Ye Xue Za Zhi. 2017. PMID: 28810324 Free PMC article. Chinese.
-
[Factors associated with early treatment response in adults with acute myeloid leukemia].Zhonghua Xue Ye Xue Za Zhi. 2017 Oct 14;38(10):869-875. doi: 10.3760/cma.j.issn.0253-2727.2017.10.009. Zhonghua Xue Ye Xue Za Zhi. 2017. PMID: 29166740 Free PMC article. Chinese.
-
The prognostic value of cytogenetics is reinforced by the kind of induction/consolidation therapy in influencing the outcome of acute myeloid leukemia--analysis of 848 patients.Leukemia. 2001 Jun;15(6):903-9. doi: 10.1038/sj.leu.2402142. Leukemia. 2001. PMID: 11417475 Review.
-
Prognosis of inv(16)/t(16;16) acute myeloid leukemia (AML): a survey of 110 cases from the French AML Intergroup.Blood. 2003 Jul 15;102(2):462-9. doi: 10.1182/blood-2002-11-3527. Epub 2003 Mar 20. Blood. 2003. PMID: 12649129 Review.
Cited by
-
Early platelet elevation after complete remission as a prognostic marker of favourable outcomes in favourable- and intermediate-risk acute myeloid leukaemia: A retrospective study.J Clin Lab Anal. 2022 Feb;36(2):e24221. doi: 10.1002/jcla.24221. Epub 2022 Jan 3. J Clin Lab Anal. 2022. PMID: 34979042 Free PMC article.
References
-
- Freireich EJ, Gehan EA, Sulman D, et al. The effect of chemotherapy on acute leukemia in the human[J] J Chronic Dis. 1961;14:593–608. - PubMed
-
- Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia[J] J Clin Oncol. 2003;21(24):4642–4649. - PubMed
-
- Larson RA, Sievers EL, Stadtmauer EA, et al. Final report of the efficacy and safety of gemtuzumab ozogamicin (Mylotarg) in patients with CD33-positive acute myeloid leukemia in first recurrence[J] Cancer. 2005;104(7):1442–1452. - PubMed
-
- de Greef GE, van Putten WL, Boogaerts M, et al. Criteria for defining a complete remission in acute myeloid leukaemia revisited. An analysis of patients treated in HOVON-SAKK co-operative group studies[J] Br J Haematol. 2005;128(2):184–191. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous