Effect of co-trimoxazole on mortality in HIV-exposed but uninfected children in Botswana (the Mpepu Study): a double-blind, randomised, placebo-controlled trial
- PMID: 28395844
- PMCID: PMC5502726
- DOI: 10.1016/S2214-109X(17)30143-2
Effect of co-trimoxazole on mortality in HIV-exposed but uninfected children in Botswana (the Mpepu Study): a double-blind, randomised, placebo-controlled trial
Abstract
Background: Co-trimoxazole prophylaxis reduces mortality among HIV-infected children, but efficacy in HIV-exposed but uninfected (HEU) children in a non-malarial, low-breastfeeding setting with a low risk of mother-to-child transmission of HIV is unclear.
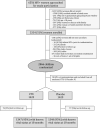
Methods: HEU children in Botswana were randomly assigned to receive co-trimoxazole (100 mg/20 mg once daily until age 6 months and 200 mg/40 mg once daily thereafter) or placebo from age 14-34 days to age 15 months. Mothers chose whether to breastfeed or formula feed their children. Breastfed children were randomly assigned to breastfeeding for 6 months (Botswana guidelines) or 12 months (WHO guidelines). The primary outcome, analysed by a modified intention-to-treat approach, was cumulative child mortality from treatment assignment to age 18 months. We also assessed HIV-free survival by duration of breastfeeding. This trial is registered with ClinicalTrials.gov, number NCT01229761.
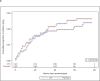
Findings: From June 7, 2011, to April 2, 2015, 2848 HEU children were randomly assigned to receive co-trimoxazole (n=1423) or placebo (n=1425). The data and safety monitoring board stopped the study early because of a low likelihood of benefit with co-trimoxazole. Only 153 (5%) children were lost to follow-up (76 in the co-trimoxazole group and 77 in the placebo group), and 2053 (72%) received treatment continuously to age 15 months, death, or study closure. Mortality after the start of study treatment was similar in the two study groups: 30 children died in the co-trimoxazole group, compared with 34 in the placebo group (estimated mortality at 18 months 2·4% vs 2·6%; difference -0·2%, 95% CI -1·5 to 1·0, p=0·70). We saw no difference in hospital admissions between groups (12·5% in the co-trimoxazole group vs 17·4% in the placebo group, p=0·19) or grade 3-4 clinical adverse events (16·5% vs 18·4%, p=0·18). Grade 3-4 anaemia did not differ between groups (8·1% vs 8·3%, p=0·93), but grade 3-4 neutropenia was more frequent in the co-trimoxazole group than in the placebo group (8·1% vs 5·8%, p=0·03). More co-trimoxazole resistance in commensal Escherichia coli isolated from stool samples was seen in children aged 3 or 6 months in the co-trimoxazole group than in the placebo group (p=0·001 and p=0·01, respectively). 572 (20%) children were breastfed. HIV infection and mortality did not differ significantly by duration of breastfeeding (3·9% for 6 months vs 1·9% for 12 months, p=0·21).
Interpretation: Prophylactic co-trimoxazole seems to offer no survival benefit among HEU children in non-malarial, low-breastfeeding areas with a low risk of mother-to-child transmission of HIV.
Funding: US National Institutes of Health.
Copyright © 2017 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
No authors have conflict of interest that could bias their work on this study or manuscript.
Figures

Comment in
-
Co-trimoxazole for HIV-exposed uninfected infants.Lancet Glob Health. 2017 May;5(5):e468-e469. doi: 10.1016/S2214-109X(17)30147-X. Lancet Glob Health. 2017. PMID: 28395832 No abstract available.
References
-
- Wiktor SZ, Sassan-Morokro M, Grant AD, et al. Efficacy of trimethoprim-sulphamethoxazole prophylaxis to decrease morbidity and mortality in HIV-1-infected patients with tuberculosis in Abidjan, Cote d’Ivoire: a randomised controlled trial. Lancet. 1999;353(9163):1469–75. - PubMed
-
- Anglaret X, Chene G, Attia A, et al. Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Cote d’Ivoire: a randomised trial. Cotrimo-CI Study Group. Lancet. 1999;353(9163):1463–8. - PubMed
-
- Mermin J, Lule J, Ekwaru JP, et al. Effect of co-trimoxazole prophylaxis on morbidity, mortality, CD4-cell count, and viral load in HIV infection in rural Uganda. Lancet. 2004;364(9443):1428–34. - PubMed
-
- Chintu C, Bhat GJ, Walker AS, et al. Co-trimoxazole as prophylaxis against opportunistic infections in HIV-infected Zambian children (CHAP): a double-blind randomised placebo-controlled trial. Lancet. 2004;364(9448):1865–71. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

