Blocking Allergic Reaction through Targeting Surface-Bound IgE with Low-Affinity Anti-IgE Antibodies
- PMID: 28396318
- PMCID: PMC6685210
- DOI: 10.4049/jimmunol.1602022
Blocking Allergic Reaction through Targeting Surface-Bound IgE with Low-Affinity Anti-IgE Antibodies
Abstract
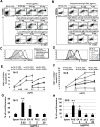
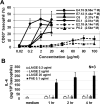
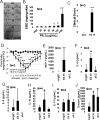
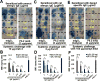
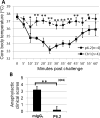
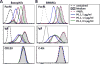
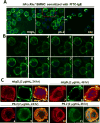
Allergic disorders have now become a major worldwide public health issue, but the effective treatment options remain limited. We report a novel approach to block allergic reactivity by targeting the surface-bound IgE of the allergic effector cells via low-affinity anti-human IgE Abs with dissociation constants in the 10-6 to 10-8 M range. We demonstrated that these low-affinity anti-IgE mAbs bind to the cell surface-bound IgE without triggering anaphylactic degranulation even at high concentration, albeit they would weakly upregulate CD203c expression on basophils. This is in contrast to the high-affinity anti-IgE mAbs that trigger anaphylactic degranulation at low concentration. Instead, the low-affinity anti-IgE mAbs profoundly block human peanut- and cat-allergic IgE-mediated basophil CD63 induction indicative of anaphylactic degranulation; suppress peanut-, cat-, and dansyl-specific IgE-mediated passive cutaneous anaphylaxis; and attenuate dansyl IgE-mediated systemic anaphylaxis in human FcεRIα transgenic mouse model. Mechanistic studies reveal that the ability of allergic reaction blockade by the low-affinity anti-IgE mAbs was correlated with their capacity to downregulate the surface IgE and FcεRI level on human basophils and the human FcεRIα transgenic mouse bone marrow-derived mast cells via driving internalization of the IgE/FcεRI complex. Our studies demonstrate that targeting surface-bound IgE with low-affinity anti-IgE Abs is capable of suppressing allergic reactivity while displaying an excellent safety profile, indicating that use of low-affinity anti-IgE mAbs holds promise as a novel therapeutic approach for IgE-mediated allergic diseases.
Copyright © 2017 by The American Association of Immunologists, Inc.
Figures



Similar articles
-
Inhibition of Allergic Reactivity through Targeting FcεRI-Bound IgE with Humanized Low-Affinity Antibodies.J Immunol. 2019 Dec 1;203(11):2777-2790. doi: 10.4049/jimmunol.1900112. Epub 2019 Oct 21. J Immunol. 2019. PMID: 31636239
-
Rapid polyclonal desensitization with antibodies to IgE and FcεRIα.J Allergy Clin Immunol. 2013 Jun;131(6):1555-64. doi: 10.1016/j.jaci.2013.02.043. Epub 2013 Apr 28. J Allergy Clin Immunol. 2013. PMID: 23632296 Free PMC article.
-
Rat monoclonal anti-murine IgE antibody removes IgE molecules already bound to mast cells or basophilic leukemia cells, resulting in the inhibition of systemic anaphylaxis and passive cutaneous anaphylaxis.Int Arch Allergy Immunol. 2002 May;128(1):24-32. doi: 10.1159/000058000. Int Arch Allergy Immunol. 2002. PMID: 12037398
-
Targeting the FcεRI Pathway as a Potential Strategy to Prevent Food-Induced Anaphylaxis.Front Immunol. 2020 Dec 17;11:614402. doi: 10.3389/fimmu.2020.614402. eCollection 2020. Front Immunol. 2020. PMID: 33391286 Free PMC article. Review.
-
Human IgE-independent systemic anaphylaxis.J Allergy Clin Immunol. 2016 Jun;137(6):1674-1680. doi: 10.1016/j.jaci.2016.02.015. Epub 2016 Apr 26. J Allergy Clin Immunol. 2016. PMID: 27130857 Free PMC article. Review.
Cited by
-
Characterization of IgE cross-reactivity and allergenicity of peanut allergens.Immunohorizons. 2025 May 30;9(7):vlaf018. doi: 10.1093/immhor/vlaf018. Immunohorizons. 2025. PMID: 40447301 Free PMC article.
-
Regulation of Trafficking and Signaling of the High Affinity IgE Receptor by FcεRIβ and the Potential Impact of FcεRIβ Splicing in Allergic Inflammation.Int J Mol Sci. 2022 Jan 12;23(2):788. doi: 10.3390/ijms23020788. Int J Mol Sci. 2022. PMID: 35054974 Free PMC article. Review.
-
Suppressing Immune Responses Using Siglec Ligand-Decorated Anti-receptor Antibodies.J Am Chem Soc. 2022 Jun 1;144(21):9302-9311. doi: 10.1021/jacs.2c00922. Epub 2022 May 20. J Am Chem Soc. 2022. PMID: 35593593 Free PMC article.
References
-
- Follenweider LM, Lambertino A. Epidemiology of asthma in the United States. Nurs Clin North Am. 2013;48:1–10. - PubMed
-
- Arbes SJ, Jr, Gergen PJ, Elliott L, Zeldin DC. Prevalences of positive skin test responses to 10 common allergens in the US population: results from the third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2005;116:377–383. - PubMed
-
- Moorman JE, Rudd RA, Johnson CA, King M, Minor P, Bailey C, Scalia MR, Akinbami LJ. National Surveillance for Asthma – United States, 1980–2004. MMWR. 2007;56:1–54. - PubMed
-
- MacGlashen DW, Jr, Bochner BS, Adelman DC, Jardieu PM, Togias A, McKenzie-White J, Sterbinsky SA, Hamilton RG, Lichtenstein L. Down-regulation of FcεRI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J Immunol. 1997;158:1438–1445. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

