Albumin, in the Presence of Calcium, Elicits a Massive Increase in Extracellular Bordetella Adenylate Cyclase Toxin
- PMID: 28396321
- PMCID: PMC5442642
- DOI: 10.1128/IAI.00198-17
Albumin, in the Presence of Calcium, Elicits a Massive Increase in Extracellular Bordetella Adenylate Cyclase Toxin
Abstract
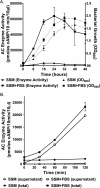
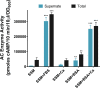
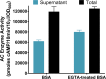
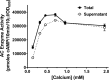
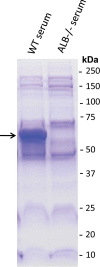
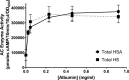
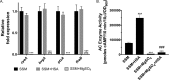
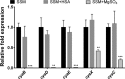
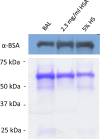
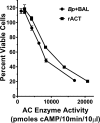
Pertussis (whooping cough), caused by Bordetella pertussis, is resurging in the United States and worldwide. Adenylate cyclase toxin (ACT) is a critical factor in establishing infection with B. pertussis and acts by specifically inhibiting the response of myeloid leukocytes to the pathogen. We report here that serum components, as discovered during growth in fetal bovine serum (FBS), elicit a robust increase in the amount of ACT, and ≥90% of this ACT is localized to the supernatant, unlike growth without FBS, in which ≥90% is associated with the bacterium. We have found that albumin, in the presence of physiological concentrations of calcium, acts specifically to enhance the amount of ACT and its localization to the supernatant. Respiratory secretions, which contain albumin, promote an increase in amount and localization of active ACT that is comparable to that elicited by serum and albumin. The response to albumin is not mediated through regulation of ACT at the transcriptional level or activation of the Bvg two-component system. As further illustration of the specificity of this phenomenon, serum collected from mice that lack albumin does not stimulate an increase in ACT. These data, demonstrating that albumin and calcium act synergistically in the host environment to increase production and release of ACT, strongly suggest that this phenomenon reflects a novel host-pathogen interaction that is central to infection with B. pertussis and other Bordetella species.
Keywords: Bordetella pertussis; RTX toxins; adenylate cyclase toxin; albumin; calcium.
Copyright © 2017 American Society for Microbiology.
Figures



Similar articles
-
Bordetella Adenylate Cyclase Toxin Elicits Airway Mucin Secretion through Activation of the cAMP Response Element Binding Protein.Int J Mol Sci. 2021 Aug 23;22(16):9064. doi: 10.3390/ijms22169064. Int J Mol Sci. 2021. PMID: 34445770 Free PMC article.
-
Pertussis toxin and adenylate cyclase toxin provide a one-two punch for establishment of Bordetella pertussis infection of the respiratory tract.Infect Immun. 2005 May;73(5):2698-703. doi: 10.1128/IAI.73.5.2698-2703.2005. Infect Immun. 2005. PMID: 15845471 Free PMC article.
-
Role of Major Toxin Virulence Factors in Pertussis Infection and Disease Pathogenesis.Adv Exp Med Biol. 2019;1183:35-51. doi: 10.1007/5584_2019_403. Adv Exp Med Biol. 2019. PMID: 31376138 Free PMC article.
-
Calcium-dependent disorder-to-order transitions are central to the secretion and folding of the CyaA toxin of Bordetella pertussis, the causative agent of whooping cough.Toxicon. 2018 Jul;149:37-44. doi: 10.1016/j.toxicon.2018.01.007. Epub 2018 Jan 12. Toxicon. 2018. PMID: 29337218 Review.
-
Bioengineering of Bordetella pertussis Adenylate Cyclase Toxin for Vaccine Development and Other Biotechnological Purposes.Toxins (Basel). 2021 Jan 22;13(2):83. doi: 10.3390/toxins13020083. Toxins (Basel). 2021. PMID: 33499260 Free PMC article. Review.
Cited by
-
Bordetella pertussis Can Be Motile and Express Flagellum-Like Structures.mBio. 2019 May 14;10(3):e00787-19. doi: 10.1128/mBio.00787-19. mBio. 2019. PMID: 31088927 Free PMC article.
-
Disrupting Bordetella Immunosuppression Reveals a Role for Eosinophils in Coordinating the Adaptive Immune Response in the Respiratory Tract.Microorganisms. 2020 Nov 17;8(11):1808. doi: 10.3390/microorganisms8111808. Microorganisms. 2020. PMID: 33212993 Free PMC article.
-
Carbapenem-resistant Acinetobacter baumannii (CRAB): metabolic adaptation and transcriptional response to human urine (HU).Res Sq [Preprint]. 2024 May 30:rs.3.rs-4415275. doi: 10.21203/rs.3.rs-4415275/v1. Res Sq. 2024. Update in: Sci Rep. 2024 Aug 19;14(1):19145. doi: 10.1038/s41598-024-70216-w. PMID: 38853891 Free PMC article. Updated. Preprint.
-
The Iron Content of Human Serum Albumin Modulates the Susceptibility of Acinetobacter baumannii to Cefiderocol.Biomedicines. 2023 Feb 20;11(2):639. doi: 10.3390/biomedicines11020639. Biomedicines. 2023. PMID: 36831178 Free PMC article.
-
Role of Albumin in Accumulation and Persistence of Tumor-Seeking Cyanine Dyes.Bioconjug Chem. 2020 Feb 19;31(2):248-259. doi: 10.1021/acs.bioconjchem.9b00771. Epub 2020 Jan 7. Bioconjug Chem. 2020. PMID: 31909595 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

