Giardia Alters Commensal Microbial Diversity throughout the Murine Gut
- PMID: 28396324
- PMCID: PMC5442636
- DOI: 10.1128/IAI.00948-16
Giardia Alters Commensal Microbial Diversity throughout the Murine Gut
Abstract
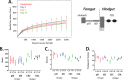
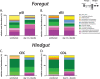
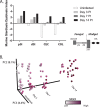
Giardia lamblia is the most frequently identified protozoan cause of intestinal infection. Over 200 million people are estimated to have acute or chronic giardiasis, with infection rates approaching 90% in areas where Giardia is endemic. Despite its significance in global health, the mechanisms of pathogenesis associated with giardiasis remain unclear, as the parasite neither produces a known toxin nor induces a robust inflammatory response. Giardia colonization and proliferation in the small intestine of the host may, however, disrupt the ecological homeostasis of gastrointestinal commensal microbes and contribute to diarrheal disease associated with giardiasis. To evaluate the impact of Giardia infection on the host microbiota, we used culture-independent methods to quantify shifts in the diversity of commensal microbes throughout the gastrointestinal tract in mice infected with Giardia We discovered that Giardia's colonization of the small intestine causes a systemic dysbiosis of aerobic and anaerobic commensal bacteria. Specifically, Giardia colonization is typified by both expansions in aerobic Proteobacteria and decreases in anaerobic Firmicutes and Melainabacteria in the murine foregut and hindgut. Based on these shifts, we created a quantitative index of murine Giardia-induced microbial dysbiosis. This index increased at all gut regions during the duration of infection, including both the proximal small intestine and the colon. Giardiasis could be an ecological disease, and the observed dysbiosis may be mediated directly via the parasite's unique anaerobic fermentative metabolism or indirectly via parasite induction of gut inflammation. This systemic alteration of murine gut commensal diversity may be the cause or the consequence of inflammatory and metabolic changes throughout the gut. Shifts in the commensal microbiota may explain observed variations in giardiasis between hosts with respect to host pathology, degree of parasite colonization, infection initiation, and eventual clearance.
Keywords: Giardia; microbiome; parasite; pathogenesis.
Copyright © 2017 American Society for Microbiology.
Figures




References
-
- Al-Mekhlafi HM, Al-Maktari MT, Jani R, Ahmed A, Anuar TS, Moktar N, Mahdy MA, Lim YA, Mahmud R, Surin J. 2013. Burden of Giardia duodenalis infection and its adverse effects on growth of schoolchildren in rural Malaysia. PLoS Negl Trop Dis 7:e2516. doi:10.1371/journal.pntd.0002516. - DOI - PMC - PubMed
-
- Furness BW, Beach MJ, Roberts JM. 2000. Giardiasis surveillance—United States, 1992-1997. MMWR CDC Surveill Summ 49:1–13. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

