Bioactivity of Oral Linaclotide in Human Colorectum for Cancer Chemoprevention
- PMID: 28396341
- PMCID: PMC5758862
- DOI: 10.1158/1940-6207.CAPR-16-0286
Bioactivity of Oral Linaclotide in Human Colorectum for Cancer Chemoprevention
Abstract
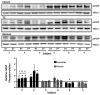
Guanylate cyclase C (GUCY2C) is a tumor-suppressing receptor silenced by loss of expression of its luminocrine hormones guanylin and uroguanylin early in colorectal carcinogenesis. This observation suggests oral replacement with a GUCY2C agonist may be an effective targeted chemoprevention agent. Linaclotide is an FDA-approved oral GUCY2C agonist formulated for gastric release, inducing fluid secretion into the small bowel to treat chronic idiopathic constipation. The ability of oral linaclotide to induce a pharmacodynamic response in epithelial cells of the colorectum in humans remains undefined. Here, we demonstrate that administration of 0.87 mg of oral linaclotide daily for 7 days to healthy volunteers, after oral colon preparation with polyethylene glycol solution (MoviPrep), activates GUCY2C, resulting in accumulation of its product cyclic (c)GMP in epithelial cells of the cecum, transverse colon, and distal rectum. GUCY2C activation by oral linaclotide was associated with homeostatic signaling, including phosphorylation of vasodilator-stimulated phosphoprotein and inhibition of proliferation quantified by reduced Ki67-positive epithelial cells. In the absence of the complete oral colonoscopy preparation, linaclotide did not alter cGMP production in epithelial cells of the colorectum, demonstrating that there was an effect related to the laxative preparation. These data show that the current FDA-approved formulation of oral linaclotide developed for small-bowel delivery to treat chronic idiopathic constipation is inadequate for reliably regulating GUCY2C in the colorectum to prevent tumorigenesis. The study results highlight the importance of developing a novel GUCY2C agonist formulated for release and activity targeted to the large intestine for colorectal cancer prevention. Cancer Prev Res; 10(6); 345-54. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
S.A. Waldman is the Chair of the Data Safety Monitoring Board for the Chart-1 Trial™ sponsored by Cardio3 Biosciences, and the Chair (uncompensated) of the Scientific Advisory Board of Targeted Diagnostics & Therapeutics, Inc., which provided research funding that, in part, supported this work and has a license to commercialize inventions related to this work. S.A. Waldman is the Samuel MV Hamilton Professor of Thomas Jefferson University. ESB and DJM received F30 Ruth Kirschstein MD-PhD Fellowship Awards.
Figures
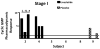


References
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. - PubMed
-
- Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, et al. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev. 2000;52:375–414. - PubMed
-
- Field M. Mechanisms of action of cholera and Escherichia coli enterotoxins. Am J Clin Nutr. 1979;32:189–96. - PubMed
-
- Guerrant RL, Hughes JM, Chang B, Robertson DC, Murad F. Activation of intestinal guanylate cyclase by heat-stable enterotoxin of Escherichia coli: studies of tissue specificity, potential receptors, and intermediates. J Infect Dis. 1980;142:220–8. - PubMed
-
- Guarino A, Cohen M, Thompson M, Dharmsathaphorn K, Giannella R. T84 cell receptor binding and guanyl cyclase activation by Escherichia coli heat-stable toxin. Am J Physiol. 1987;253:G775–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

