Increased maternal and fetal cholesterol efflux capacity and placental CYP27A1 expression in preeclampsia
- PMID: 28396342
- PMCID: PMC5454503
- DOI: 10.1194/jlr.M071985
Increased maternal and fetal cholesterol efflux capacity and placental CYP27A1 expression in preeclampsia
Abstract
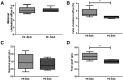
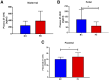
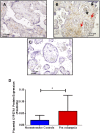
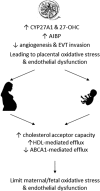
Preeclampsia is a pregnancy-specific condition that leads to increased cardiovascular risk in later life. A decrease in cholesterol efflux capacity is linked to CVD. We hypothesized that in preeclampsia there would be a disruption of maternal/fetal plasma to efflux cholesterol, as well as differences in the concentrations of both placental sterol 27-hydroxylase (CYP27A1) and apoA1 binding protein (AIBP). Total, HDL-, and ABCA1-mediated cholesterol effluxes were performed with maternal and fetal plasma from women with preeclampsia and normotensive controls (both n = 17). apoA1 and apoE were quantified by chemiluminescence, and 27-hydroxycholesterol (27-OHC) by GC-MS. Immunohistochemistry was used to determine placental expression/localization of CYP27A1, AIBP, apoA1, apoE, and SRB1. Maternal and fetal total and HDL-mediated cholesterol efflux capacities were increased in preeclampsia (by 10-20%), but ABCA1-mediated efflux was decreased (by 20-35%; P < 0.05). Maternal and fetal apoE concentrations were higher in preeclampsia. Fetal plasma 27-OHC levels were decreased in preeclamptic samples (P < 0.05). Placental protein expression of both CYP27A1 and AIBP were localized around fetal vessels and significantly increased in preeclampsia (P = 0.04). Placental 27-OHC concentrations were also raised in preeclampsia (P < 0.05). Increased HDL-mediated cholesterol efflux capacity and placental CYP27A1/27-OHC could be a rescue mechanism in preeclampsia, to remove cholesterol from cells to limit lipid peroxidation and increase placental angiogenesis.
Keywords: 27-hydroxycholesterol; apolipoproteins; hypertension; lipid/efflux; pregnancy; sterol 27-hydroxylase.
Copyright © 2017 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Ghulmiyyah L., and Sibai B.. 2012. Maternal mortality from preeclampsia/eclampsia. Semin. Perinatol. 36: 56–59. - PubMed
-
- Duley L. 2009. The global impact of pre-eclampsia and eclampsia. Semin. Perinatol. 33: 130–137. - PubMed
-
- Brown M. C., Best K. E., Pearce M. S., Waugh J., Robson S. C., and Bell R.. 2013. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur. J. Epidemiol. 28: 1–19. - PubMed
-
- Mosca L., Benjamin E. J., Berra K., Bezanson J. L., Dolor R. J., Lloyd-Jones D. M., Newby L. K., Pina I. L., Roger V. L., Shaw L. J., et al. 2011. Effectiveness-based guidelines for the prevention of cardiovascular disease in women–2011 update: a guideline from the American Heart Association. J. Am. Coll. Cardiol. 57: 1404–1423. - PMC - PubMed
-
- Hubel C. A. 1999. Oxidative stress in the pathogenesis of preeclampsia. Proc. Soc. Exp. Biol. Med. 222: 222–235. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

