Multifunctional Telodendrimer Nanocarriers Restore Synergy of Bortezomib and Doxorubicin in Ovarian Cancer Treatment
- PMID: 28396359
- PMCID: PMC5478165
- DOI: 10.1158/0008-5472.CAN-16-3119
Multifunctional Telodendrimer Nanocarriers Restore Synergy of Bortezomib and Doxorubicin in Ovarian Cancer Treatment
Abstract
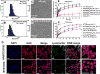
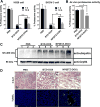
We have developed multifunctional nanoparticles for codelivery of bortezomib and doxorubicin to synchronize their pharmacokinetic profiles and synergize their activities in solid tumor treatment, a need still unmet in the clinic. Micellar nanoparticles were formed by a spatially segregated, linear-dendritic telodendrimer containing three segments: a hydrophilic polyethylene glycol (PEG), a bortezomib-conjugating intermediate, and a dendritic doxorubicin-affinitive interior. Bortezomib-conjugated telodendrimers, together with doxorubicin, self-assembled into monodispersed micelles [NP(BTZ-DOX)] with small particle sizes (20-30 nm) for dual drug delivery. NP(BTZ-DOX) displayed excellent drug-loading capacity and stability, which minimized premature drug leakage and synchronized drug release profiles. Bortezomib release was accelerated significantly by acidic pH, facilitating drug availability in the acidic tumor microenvironment. Synergistic anticancer effects of combined bortezomib and doxorubicin were observed in vitro against both multiple myeloma and ovarian cancer cells. NP(BTZ-DOX) prolonged payload circulation and targeted tumors in vivo efficiently with superior signal ratios of tumor to normal organs. In vitro and in vivo proteasome inhibition analysis and biodistribution studies revealed decreased toxicity and efficient intratumoral bortezomib and doxorubicin delivery by nanoformulation. NP(BTZ-DOX) exhibited significantly improved ovarian cancer treatment in SKOV-3 xenograft mouse models in comparison with free drugs and their combinations, including bortezomib and Doxil. In summary, tumor-targeted and synchronized delivery system elicits enhanced anticancer effects and merits further development in the clinical setting. Cancer Res; 77(12); 3293-305. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
Figures



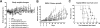
References
-
- Foley OW, Rauh-Hain JA, del Carmen MG. Recurrent epithelial ovarian cancer: an update on treatment. Oncology (Williston Park) 2013;27:288–94. 98. - PubMed
-
- Gatti L, Zuco V, Zaffaroni N, Perego P. Drug combinations with proteasome inhibitors in antitumor therapy. Current pharmaceutical design. 2013;19:4094–114. - PubMed
-
- Gottesman MM. Mechanisms of cancer drug resistance. Annual review of medicine. 2002;53:615–27. - PubMed
-
- Longley D, Johnston P. Molecular mechanisms of drug resistance. The Journal of pathology. 2005;205:275–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

