Mechanosensation is evolutionarily tuned to locomotor mechanics
- PMID: 28396411
- PMCID: PMC5410822
- DOI: 10.1073/pnas.1616839114
Mechanosensation is evolutionarily tuned to locomotor mechanics
Abstract
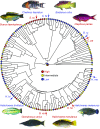
The biomechanics of animal limbs has evolved to meet the functional demands for movement associated with different behaviors and environments. Effective movement relies not only on limb mechanics but also on appropriate mechanosensory feedback. By comparing sensory ability and mechanics within a phylogenetic framework, we show that peripheral mechanosensation has evolved with limb biomechanics, evolutionarily tuning the neuromechanical system to its functional demands. We examined sensory physiology and mechanics of the pectoral fins, forelimb homologs, in the fish family Labridae. Labrid fishes exhibit extraordinary morphological and behavioral diversity and use pectoral fin-based propulsion with fins ranging in shape from high aspect ratio (AR) wing-like fins to low AR paddle-like fins. Phylogenetic character analysis demonstrates that high AR fins evolved independently multiple times in this group. Four pairs of species were examined; each included a plesiomorphic low AR and a high AR species. Within each species pair, the high AR species demonstrated significantly stiffer fin rays in comparison with the low AR species. Afferent sensory nerve activity was recorded during fin ray bending. In all cases, afferents of stiffer fins were more sensitive at lower displacement amplitudes, demonstrating mechanosensory tuning to fin mechanics and a consistent pattern of correlated evolution. We suggest that these data provide a clear example of parallel evolution in a complex neuromechanical system, with a strong link between multiple phenotypic characters: pectoral fin shape, swimming behavior, fin ray stiffness, and mechanosensory sensitivity.
Keywords: Labridae; evolution; locomotion; mechanosensation; neuromechanics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Fins as Mechanosensors for Movement and Touch-Related Behaviors.Integr Comp Biol. 2018 Nov 1;58(5):844-859. doi: 10.1093/icb/icy065. Integr Comp Biol. 2018. PMID: 29917043 Review.
-
Pectoral fin kinematics and motor patterns are shaped by fin ray mechanosensation during steady swimming in Scarus quoyi.J Exp Biol. 2020 Jan 23;223(Pt 2):jeb211466. doi: 10.1242/jeb.211466. J Exp Biol. 2020. PMID: 31862848
-
The relationship between pectoral fin ray stiffness and swimming behavior in Labridae: insights into design, performance and ecology.J Exp Biol. 2018 Jan 9;221(Pt 1):jeb163360. doi: 10.1242/jeb.163360. J Exp Biol. 2018. PMID: 29162638
-
Performance limits of labriform propulsion and correlates with fin shape and motion.J Exp Biol. 2002 Jan;205(Pt 2):177-87. doi: 10.1242/jeb.205.2.177. J Exp Biol. 2002. PMID: 11821484
-
The Water to Land Transition Submerged: Multifunctional Design of Pectoral Fins for Use in Swimming and in Association with Underwater Substrate.Integr Comp Biol. 2022 Oct 29;62(4):908-921. doi: 10.1093/icb/icac061. Integr Comp Biol. 2022. PMID: 35652788 Free PMC article. Review.
Cited by
-
Comparative Principles for Next-Generation Neuroscience.Front Behav Neurosci. 2019 Feb 5;13:12. doi: 10.3389/fnbeh.2019.00012. eCollection 2019. Front Behav Neurosci. 2019. PMID: 30787871 Free PMC article.
-
Knowing when to stick: touch receptors found in the remora adhesive disc.R Soc Open Sci. 2020 Jan 15;7(1):190990. doi: 10.1098/rsos.190990. eCollection 2020 Jan. R Soc Open Sci. 2020. PMID: 32218935 Free PMC article.
-
Mechanical Properties of a Drosophila Larval Chordotonal Organ.Biophys J. 2017 Dec 19;113(12):2796-2804. doi: 10.1016/j.bpj.2017.08.061. Biophys J. 2017. PMID: 29262372 Free PMC article.
-
Hindbrain and Spinal Cord Contributions to the Cutaneous Sensory Innervation of the Larval Zebrafish Pectoral Fin.Front Neuroanat. 2020 Oct 20;14:581821. doi: 10.3389/fnana.2020.581821. eCollection 2020. Front Neuroanat. 2020. PMID: 33192344 Free PMC article.
-
Distribution and Restoration of Serotonin-Immunoreactive Paraneuronal Cells During Caudal Fin Regeneration in Zebrafish.Front Mol Neurosci. 2019 Sep 19;12:227. doi: 10.3389/fnmol.2019.00227. eCollection 2019. Front Mol Neurosci. 2019. PMID: 31616250 Free PMC article.
References
-
- Gillis GB, Blob RW. How muscles accommodate movement in different physical environments: Aquatic vs. terrestrial locomotion in vertebrates. Comp Biochem Physiol A Mol Integr Physiol. 2001;131:61–75. - PubMed
-
- Losos JB. Ecomorphology, performance capability, and scaling of West-Indian anolis lizards: An evolutionary analysis. Ecol Monogr. 1990;60:369–388.
-
- Wootton RJ. Functional morphology of insect wings. Annu Rev Entomol. 1992;37:113–140.
-
- Bellwood DR, Wainwright PC. Locomotion in labrid fishes: Implications for habitat use and cross-shelf biogeography on the Great Barrier Reef. Coral Reefs. 2001;20:139–150.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

