Enterococcus faecalis bacteriocin EntV inhibits hyphal morphogenesis, biofilm formation, and virulence of Candida albicans
- PMID: 28396417
- PMCID: PMC5410809
- DOI: 10.1073/pnas.1620432114
Enterococcus faecalis bacteriocin EntV inhibits hyphal morphogenesis, biofilm formation, and virulence of Candida albicans
Abstract
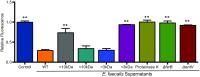
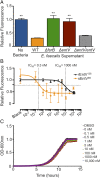
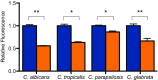
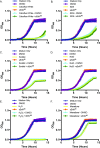
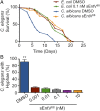
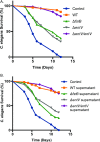
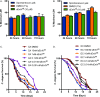
Enterococcus faecalis, a Gram-positive bacterium, and Candida albicans, a fungus, occupy overlapping niches as ubiquitous constituents of the gastrointestinal and oral microbiome. Both species also are among the most important and problematic, opportunistic nosocomial pathogens. Surprisingly, these two species antagonize each other's virulence in both nematode infection and in vitro biofilm models. We report here the identification of the E. faecalis bacteriocin, EntV, produced from the entV (ef1097) locus, as both necessary and sufficient for the reduction of C. albicans virulence and biofilm formation through the inhibition of hyphal formation, a critical virulence trait. A synthetic version of the mature 68-aa peptide potently blocks biofilm development on solid substrates in multiple media conditions and disrupts preformed biofilms, which are resistant to current antifungal agents. EntV68 is protective in three fungal infection models at nanomolar or lower concentrations. First, nematodes treated with the peptide at 0.1 nM are completely resistant to killing by C. albicans The peptide also protects macrophages and augments their antifungal activity. Finally, EntV68 reduces epithelial invasion, inflammation, and fungal burden in a murine model of oropharyngeal candidiasis. In all three models, the peptide greatly reduces the number of fungal cells present in the hyphal form. Despite these profound effects, EntV68 has no effect on C. albicans viability, even in the presence of significant host-mimicking stresses. These findings demonstrate that EntV has potential as an antifungal agent that targets virulence rather than viability.
Keywords: Candida albicans; Enterococcus faecalis; bacteriocins; biofilms.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Agudelo Higuita NI, Huycke MM. Enterococcal disease, epidemiology, and implications for treatment. In: Gilmore MS, Clewell DB, Ike Y, Shankar N, editors. Enterococci: From Commensals to Leading Causes of Drug Resistant Infection. Mass Eye Ear Infirmary; Boston: 2014.
-
- Lebreton F, Willems RJL, Gilmore MS. Enterococcus diversity, origins in nature, and gut colonization. In: Gilmore MS, Clewell DB, Ike Y, Shankar N, editors. Enterococci: From Commensals to Leading Causes of Drug Resistant Infection. Mass Eye Ear Infirmary; Boston: 2014. - PubMed
-
- Moran G, Coleman D, Sullivan D. 2012. An introduction to the medically relevant Candida species. Candida and Candidiasis, eds Calderone RA, Clancy CJ (Am Soc Microbiol, Washington), pp 11–26. - PubMed
-
- Oever JT, Netea MG. The bacteriome-mycobiome interaction and antifungal host defense. Eur J Immunol. 2014;44:3182–3191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

