Formation of nucleobases in a Miller-Urey reducing atmosphere
- PMID: 28396441
- PMCID: PMC5410828
- DOI: 10.1073/pnas.1700010114
Formation of nucleobases in a Miller-Urey reducing atmosphere
Abstract
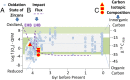
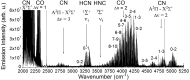
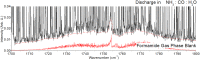
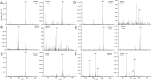
The Miller-Urey experiments pioneered modern research on the molecular origins of life, but their actual relevance in this field was later questioned because the gas mixture used in their research is considered too reducing with respect to the most accepted hypotheses for the conditions on primordial Earth. In particular, the production of only amino acids has been taken as evidence of the limited relevance of the results. Here, we report an experimental work, combined with state-of-the-art computational methods, in which both electric discharge and laser-driven plasma impact simulations were carried out in a reducing atmosphere containing NH3 + CO. We show that RNA nucleobases are synthesized in these experiments, strongly supporting the possibility of the emergence of biologically relevant molecules in a reducing atmosphere. The reconstructed synthetic pathways indicate that small radicals and formamide play a crucial role, in agreement with a number of recent experimental and theoretical results.
Keywords: asteroid impact; life origins; reducing atmosphere.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



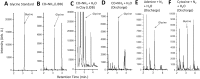
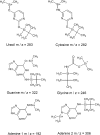

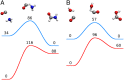
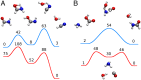

References
-
- Miller SL. A production of amino acids under possible primitive Earth conditions. Science. 1953;117:528–529. - PubMed
-
- Wollrab E, et al. Chemical analysis of a “Miller-type” complex prebiotic broth: Part I: Chemical diversity, oxygen and nitrogen based polymers. Orig Life Evol Biosph. 2016;46:149–169. - PubMed
-
- McCollom TM. Miller–Urey and beyond: What have we learned about prebiotic organic synthesis reactions in the past 60 years? Annu Rev Earth Planet Sci. 2013;41:207–229.
-
- Johnson A, Cleaves HJ, Bada JL, Lazcano A. The diversity of the original prebiotic soup: Re-analyzing the original Miller–Urey spark discharge experiments. Orig Life Evol Biosph. 2009;39:240–241.
-
- Johnson AP, et al. The Miller volcanic spark discharge experiment. Science. 2008;322:404. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

