Adipocyte Metabolic Pathways Regulated by Diet Control the Female Germline Stem Cell Lineage in Drosophila melanogaster
- PMID: 28396508
- PMCID: PMC5499197
- DOI: 10.1534/genetics.117.201921
Adipocyte Metabolic Pathways Regulated by Diet Control the Female Germline Stem Cell Lineage in Drosophila melanogaster
Abstract
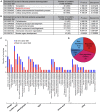
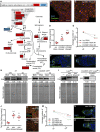
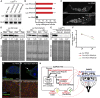
Nutrients affect adult stem cells through complex mechanisms involving multiple organs. Adipocytes are highly sensitive to diet and have key metabolic roles, and obesity increases the risk for many cancers. How diet-regulated adipocyte metabolic pathways influence normal stem cell lineages, however, remains unclear. Drosophila melanogaster has highly conserved adipocyte metabolism and a well-characterized female germline stem cell (GSC) lineage response to diet. Here, we conducted an isobaric tags for relative and absolute quantification (iTRAQ) proteomic analysis to identify diet-regulated adipocyte metabolic pathways that control the female GSC lineage. On a rich (relative to poor) diet, adipocyte Hexokinase-C and metabolic enzymes involved in pyruvate/acetyl-CoA production are upregulated, promoting a shift of glucose metabolism toward macromolecule biosynthesis. Adipocyte-specific knockdown shows that these enzymes support early GSC progeny survival. Further, enzymes catalyzing fatty acid oxidation and phosphatidylethanolamine synthesis in adipocytes promote GSC maintenance, whereas lipid and iron transport from adipocytes controls vitellogenesis and GSC number, respectively. These results show a functional relationship between specific metabolic pathways in adipocytes and distinct processes in the GSC lineage, suggesting the adipocyte metabolism-stem cell link as an important area of investigation in other stem cell systems.
Keywords: Drosophila; adipocytes; germline; metabolism; oogenesis; stem cells.
Copyright © 2017 by the Genetics Society of America.
Figures




Similar articles
-
Insulin signaling acts in adult adipocytes via GSK-3β and independently of FOXO to control Drosophila female germline stem cell numbers.Dev Biol. 2018 Aug 1;440(1):31-39. doi: 10.1016/j.ydbio.2018.04.028. Epub 2018 May 2. Dev Biol. 2018. PMID: 29729259 Free PMC article.
-
Regulation of adult female germline stem cells by nutrient-responsive signaling.Curr Opin Insect Sci. 2020 Feb;37:16-22. doi: 10.1016/j.cois.2019.10.005. Epub 2020 Jan 30. Curr Opin Insect Sci. 2020. PMID: 32070932 Review.
-
Chromatin and gene expression changes during female Drosophila germline stem cell development illuminate the biology of highly potent stem cells.Elife. 2023 Oct 13;12:RP90509. doi: 10.7554/eLife.90509. Elife. 2023. PMID: 37831064 Free PMC article.
-
Insulin-independent role of adiponectin receptor signaling in Drosophila germline stem cell maintenance.Dev Biol. 2015 Mar 15;399(2):226-36. doi: 10.1016/j.ydbio.2014.12.033. Epub 2015 Jan 7. Dev Biol. 2015. PMID: 25576925 Free PMC article.
-
Local and Physiological Control of Germline Stem Cell Lineages in Drosophila melanogaster.Genetics. 2019 Sep;213(1):9-26. doi: 10.1534/genetics.119.300234. Genetics. 2019. PMID: 31488592 Free PMC article. Review.
Cited by
-
The Nuclear Receptor Seven Up Regulates Genes Involved in Immunity and Xenobiotic Response in the Adult Drosophila Female Fat Body.G3 (Bethesda). 2020 Dec 3;10(12):4625-4635. doi: 10.1534/g3.120.401745. G3 (Bethesda). 2020. PMID: 33087412 Free PMC article.
-
Untargeted Lipidomics Analysis Unravels the Different Metabolites in the Fat Body of Mated Bumblebee (Bombus terrestris) Queens.Int J Mol Sci. 2023 Oct 21;24(20):15408. doi: 10.3390/ijms242015408. Int J Mol Sci. 2023. PMID: 37895088 Free PMC article.
-
Regulation of Mating-Induced Increase in Female Germline Stem Cells in the Fruit Fly Drosophila melanogaster.Front Physiol. 2021 Dec 7;12:785435. doi: 10.3389/fphys.2021.785435. eCollection 2021. Front Physiol. 2021. PMID: 34950056 Free PMC article. Review.
-
A high-sugar diet, but not obesity, reduces female fertility in Drosophila melanogaster.Development. 2023 Oct 15;150(20):dev201769. doi: 10.1242/dev.201769. Epub 2023 Oct 16. Development. 2023. PMID: 37795747 Free PMC article.
-
The effect of Wolbachia on gene expression in Drosophila paulistorum and its implications for symbiont-induced host speciation.BMC Genomics. 2019 Jun 7;20(1):465. doi: 10.1186/s12864-019-5816-9. BMC Genomics. 2019. PMID: 31174466 Free PMC article.
References
-
- Allen A. K., Spradling A. C., 2008. The Sf1-related nuclear hormone receptor Hr39 regulates Drosophila female reproductive tract development and function. Development 135: 311–321. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

