Database-augmented Mass Spectrometry Analysis of Exosomes Identifies Claudin 3 as a Putative Prostate Cancer Biomarker
- PMID: 28396511
- PMCID: PMC5461549
- DOI: 10.1074/mcp.M117.068577
Database-augmented Mass Spectrometry Analysis of Exosomes Identifies Claudin 3 as a Putative Prostate Cancer Biomarker
Abstract
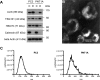
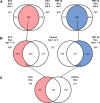
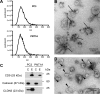
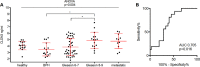
In prostate cancer and other malignancies sensitive and robust biomarkers are lacking or have relevant limitations. Prostate specific antigen (PSA), the only biomarker widely used in prostate cancer, is suffering from low specificity. Exosomes offer new perspectives in the discovery of blood-based biomarkers. Here we present a proof-of principle study for a proteomics-based identification pipeline, implementing existing data sources, to exemplarily identify exosome-based biomarker candidates in prostate cancer.Exosomes from malignant PC3 and benign PNT1A cells and from FBS-containing medium were isolated using sequential ultracentrifugation. Exosome and control samples were analyzed on an LTQ-Orbitrap XL mass spectrometer. Proteomic data is available via ProteomeXchange with identifier PXD003651. We developed a scoring scheme to rank 64 proteins exclusively found in PC3 exosomes, integrating data from four public databases and published mass spectrometry data sets. Among the top candidates, we focused on the tight junction protein claudin 3. Retests under serum-free conditions using immunoblotting and immunogold labeling confirmed the presence of claudin 3 on PC3 exosomes. Claudin 3 levels were determined in the blood plasma of patients with localized (n = 58; 42 with Gleason score 6-7, 16 with Gleason score ≥8) and metastatic prostate cancer (n = 11) compared with patients with benign prostatic hyperplasia (n = 15) and healthy individuals (n = 15) using ELISA, without prior laborious exosome isolation. ANOVA showed different CLDN3 plasma levels in these groups (p = 0.004). CLDN3 levels were higher in patients with Gleason ≥8 tumors compared with patients with benign prostatic hyperplasia (p = 0.012) and Gleason 6-7 tumors (p = 0.029). In patients with localized tumors CLDN3 levels predicted a Gleason score ≥ 8 (AUC = 0.705; p = 0.016) and did not correlate with serum PSA.By using the described workflow claudin 3 was identified and validated as a potential blood-based biomarker in prostate cancer. Furthermore this workflow could serve as a template to be used in other cancer entities.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
Similar articles
-
Plasma-derived exosomal survivin, a plausible biomarker for early detection of prostate cancer.PLoS One. 2012;7(10):e46737. doi: 10.1371/journal.pone.0046737. Epub 2012 Oct 16. PLoS One. 2012. PMID: 23091600 Free PMC article.
-
Investigation on core-fucosylated prostate-specific antigen as a refined biomarker for differentiation of benign prostate hyperplasia and prostate cancer of different aggressiveness.Tumour Biol. 2019 Mar;41(3):1010428319827223. doi: 10.1177/1010428319827223. Tumour Biol. 2019. PMID: 30907281
-
Gamma-glutamyltransferase activity in exosomes as a potential marker for prostate cancer.BMC Cancer. 2017 May 5;17(1):316. doi: 10.1186/s12885-017-3301-x. BMC Cancer. 2017. PMID: 28476099 Free PMC article.
-
Proteomics in diagnosis of prostate cancer.Pril (Makedon Akad Nauk Umet Odd Med Nauki). 2015;36(1):5-36. Pril (Makedon Akad Nauk Umet Odd Med Nauki). 2015. PMID: 26076772 Review.
-
Biomarkers in Prostate Cancer Diagnosis: From Current Knowledge to the Role of Metabolomics and Exosomes.Int J Mol Sci. 2021 Apr 22;22(9):4367. doi: 10.3390/ijms22094367. Int J Mol Sci. 2021. PMID: 33922033 Free PMC article. Review.
Cited by
-
Extracellular vesicles and particles impact the systemic landscape of cancer.EMBO J. 2022 Sep 15;41(18):e109288. doi: 10.15252/embj.2021109288. Epub 2022 Sep 2. EMBO J. 2022. PMID: 36052513 Free PMC article. Review.
-
Challenges and Opportunities in Clinical Applications of Blood-Based Proteomics in Cancer.Cancers (Basel). 2020 Aug 27;12(9):2428. doi: 10.3390/cancers12092428. Cancers (Basel). 2020. PMID: 32867043 Free PMC article. Review.
-
Claudin-3 Loss of Expression Is a Prognostic Marker in Castration-Resistant Prostate Cancer.Int J Mol Sci. 2023 Jan 2;24(1):803. doi: 10.3390/ijms24010803. Int J Mol Sci. 2023. PMID: 36614243 Free PMC article.
-
Molecular mechanisms and clinical applications of exosomes in prostate cancer.Biomark Res. 2022 Jul 29;10(1):56. doi: 10.1186/s40364-022-00398-w. Biomark Res. 2022. PMID: 35906674 Free PMC article. Review.
-
"Exosomics"-A Review of Biophysics, Biology and Biochemistry of Exosomes With a Focus on Human Breast Milk.Front Genet. 2018 Mar 27;9:92. doi: 10.3389/fgene.2018.00092. eCollection 2018. Front Genet. 2018. PMID: 29636770 Free PMC article. Review.
References
-
- Jemal A., Bray F., Center M. M., Ferlay J., Ward E., and Forman D. (2011) Global cancer statistics. CA. Cancer J. Clin. 61, 69–90 - PubMed
-
- Mottet N., Bellmunt J., Bolla M., Joniau S., Mason M., Matveev V., Schmid H.-P., Van der Kwast T., Wiegel T., Zattoni F., and Heidenreich A. (2011) EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur. Urol. 59, 572–583 - PubMed
-
- Yap T. A., Zivi A., Omlin A., and de Bono J. S. (2011) The changing therapeutic landscape of castration-resistant prostate cancer. Nat. Rev. Clin. Oncol. 8, 597–610 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

