Synthetic (p)ppGpp Analogue Is an Inhibitor of Stringent Response in Mycobacteria
- PMID: 28396544
- PMCID: PMC5444170
- DOI: 10.1128/AAC.00443-17
Synthetic (p)ppGpp Analogue Is an Inhibitor of Stringent Response in Mycobacteria
Abstract
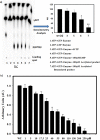
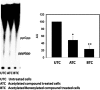
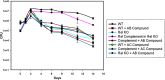
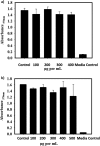
Bacteria elicit an adaptive response against hostile conditions such as starvation and other kinds of stresses. Their ability to survive such conditions depends, in part, on stringent response pathways. (p)ppGpp, considered to be the master regulator of the stringent response, is a novel target for inhibiting the survival of bacteria. In mycobacteria, the (p)ppGpp synthetase activity of bifunctional Rel is critical for stress response and persistence inside a host. Our aim was to design an inhibitor of (p)ppGpp synthesis, monitor its efficiency using enzyme kinetics, and assess its phenotypic effects in mycobacteria. As such, new sets of inhibitors targeting (p)ppGpp synthesis were synthesized and characterized by mass spectrometry and nuclear magnetic resonance spectroscopy. We observed significant inhibition of (p)ppGpp synthesis by RelMsm in the presence of designed inhibitors in a dose-dependent manner, which we further confirmed by monitoring the enzyme kinetics. The Rel enzyme inhibitor binding kinetics were investigated by isothermal titration calorimetry. Subsequently, the effects of the compounds on long-term persistence, biofilm formation, and biofilm disruption were assayed in Mycobacterium smegmatis, where inhibition in each case was observed. In vivo, (p)ppGpp levels were found to be downregulated in M. smegmatis treated with the synthetic inhibitors. The compounds reported here also inhibited biofilm formation by the pathogen Mycobacterium tuberculosis The compounds were tested for toxicity by using an MTT assay with H460 cells and a hemolysis assay with human red blood cells, for which they were found to be nontoxic. The permeability of compounds across the cell membrane of human lung epithelial cells was also confirmed by mass spectrometry.
Keywords: (p)ppGpp; mycobacteria; stringent response.
Copyright © 2017 American Society for Microbiology.
Figures

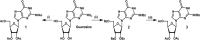






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

