Tollip Deficiency Alters Atherosclerosis and Steatosis by Disrupting Lipophagy
- PMID: 28396568
- PMCID: PMC5532987
- DOI: 10.1161/JAHA.116.004078
Tollip Deficiency Alters Atherosclerosis and Steatosis by Disrupting Lipophagy
Abstract
Background: Compromised lipophagy with unknown mechanisms may be critically involved in the intracellular accumulation of lipids, contributing to elevated atherosclerosis and liver steatosis. We hypothesize that toll-interacting protein (Tollip), a key innate immune molecule involved in the fusion of autolysosome, may play a significant role in lipophagy and modulate lipid accumulation during the pathogenesis of atherosclerosis and liver steatosis.
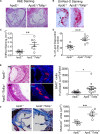
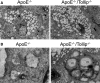
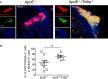
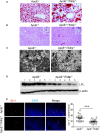
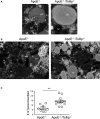
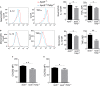
Methods and results: By comparing mice fed with either a Western high-fat diet or a regular chow diet, we observed that both atherosclerosis and liver steatosis were aggravated in apolipoprotein E-deficient (ApoE-/-)/Tollip-/- mice as compared with ApoE-/- mice. Through electron microscopy analyses, we observed compromised fusion of lipid droplets with lysosomes within aortic macrophages as well as liver hepatocytes from ApoE-/-/Tollip-/- mice as compared with ApoE-/- mice. As a molecular indicator for disrupted lysosome fusion, the levels of p62 were significantly elevated in aortic and liver tissues from ApoE-/-/Tollip-/- mice. Molecules involved in facilitating lipophagy completion such as Ras-related protein 7 and gamma-aminobutyric acid receptor-associated protein were reduced in ApoE-/-/Tollip-/- mice as compared with ApoE-/- mice. Intriguingly, ApoE-/-/Tollip-/- mice had reduced circulating levels of inflammatory cytokines such as tumor necrosis factor-α and increased levels of transforming growth factor-β. The reduced inflammation due to Tollip deficiency is consistent with a stable atherosclerotic plaque phenotype with increased levels of plaque collagen and smooth muscle cells in ApoE-/-/Tollip-/- mice.
Conclusions: Tollip deficiency selectively leads to enlarged yet stable atherosclerotic plaques, increased circulating lipids, liver steatosis, and reduced inflammation. Compromised lipophagy and reduced expression of inflammatory mediators due to Tollip deficiency may be the underlying causes. Our data suggest that lipid accumulation and inflammation may be intertwined yet independent processes during the progression of atherosclerosis and steatosis.
Keywords: Tollip; animal model cardiovascular disease; atherosclerosis; hyperlipidemia; inflammation; lipophagy.
© 2017 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley.
Figures


References
-
- Braunwald E. Shattuck lecture–cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med. 1997;337:1360–1369. - PubMed
-
- Burns K, Clatworthy J, Martin L, Martinon F, Plumpton C, Maschera B, Lewis A, Ray K, Tschopp J, Volpe F. Tollip, a new component of the IL‐1RI pathway, links IRAK to the IL‐1 receptor. Nat Cell Biol. 2000;2:346–351. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

