Sphingosine kinase 1-interacting protein is a novel regulator of glucose-stimulated insulin secretion
- PMID: 28396589
- PMCID: PMC5429731
- DOI: 10.1038/s41598-017-00900-7
Sphingosine kinase 1-interacting protein is a novel regulator of glucose-stimulated insulin secretion
Abstract
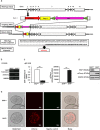
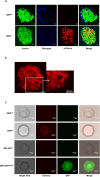
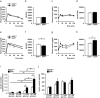
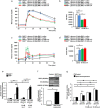
Glucose-stimulated insulin secretion (GSIS) is essential in keeping blood glucose levels within normal range. GSIS is impaired in type 2 diabetes, and its recovery is crucial in treatment of the disease. We find here that sphingosine kinase 1-interacting protein (SKIP, also called Sphkap) is highly expressed in pancreatic β-cells but not in α-cells. Intraperitoneal glucose tolerance test showed that plasma glucose levels were decreased and insulin levels were increased in SKIP-/- mice compared to SKIP+/+ mice, but exendin-4-enhanced insulin secretion was masked. GSIS was amplified more in SKIP-/- but exendin-4-enhanced insulin secretion was masked compared to that in SKIP+/+ islets. The ATP and cAMP content were similarly increased in SKIP+/+ and SKIP-/- islets; depolarization-evoked, PKA and cAMP-mediated insulin secretion were not affected. Inhibition of PDE activity equally augmented GSIS in SKIP+/+ and SKIP-/- islets. These results indicate that SKIP modulates GSIS by a pathway distinct from that of cAMP-, PDE- and sphingosine kinase-dependent pathways.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

